The US Food and Drug Administration (FDA) yesterday approved the first recombinant coagulation factor IX (Rixubis, Baxter Healthcare) for the routine prevention of bleeding in patients with hemophilia B who are 16 years of age and older, the agency announced.
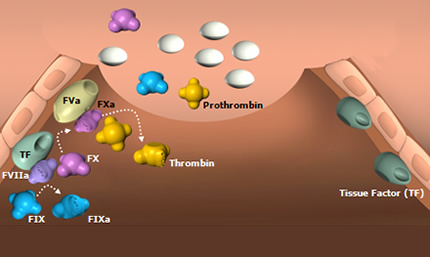
Hemophilia B is a rare genetic disorder that is also known as factor IX deficiency because patients have insufficient levels of the factor IX blood-clotting protein. It is also sometimes called Christmas disease because researchers first identified it — and distinguished it from the more common hemophilia A — in a boy named Stephen Christmas in 1952. Hemophilia B can produce potentially serious bleeding, especially into joints, which can be destroyed in the process.
"Rixubis becomes a new weapon in our arsenal to protect hemophilia B patients," said Karen Midthun, MD, director of the FDA's Center for Biologics Evaluation and Research, in a news release. "This approval provides patients and physicians with an alternative treatment option to prevent or reduce the frequency of bleeding episodes."
In addition to routine prophylaxis, the new drug is indicated for the control of bleeding episodes as well as management during hospitalization for surgery, according to the news release.
A purified protein created through recombinant DNA technology, the drug comes in single-use vials of freeze-dried powder. It is injected intravenously once it is reconstituted with sterile water. For routine prophylaxis, it is administered twice a week.
The FDA based its approval of Rixubis on a multicenter study of 73 males aged 12 years and older who received the drug for routine prophylaxis or control of bleeding episodes as needed. Patients taking Rixubis on a prophylactic basis had a 75% lower annual rate of bleeding compared with patients who have historically received on-demand therapy.
Distorted taste, pain in an extremity, and atypical blood test results were the adverse events most commonly observed in patients in clinical studies. A less common adverse event is life-threatening anaphylaxis.
Because only 3300 individuals in the United States have hemophilia B, Rixubis qualified for the FDA's orphan-drug status, which gives manufacturers economic benefits that make commercializing a drug for a small number of patients more feasible.
More information about yesterday's FDA approval of Rixubis is available on the agency's Web site.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 