Edouard Vannier, Ph.D., and Peter J. Krause, M.D.
N Engl J Med 2012; 366:2397-2407June 21, 2012
Human babesiosis is an infectious disease caused by intraerythrocytic protozoa of the genus babesia. The disease is named after Victor Babes, the Hungarian pathologist and microbiologist who identified intraerythrocytic microorganisms as the cause of febrile hemoglobinuria in cattle in 1888.1 Five years later, Theobald Smith and Frederick L. Kilborne identified a tick as the vector for transmission of Babesia bigemina in Texas cattle.2 This seminal observation established for the first time that an arthropod could transmit an infectious agent to a vertebrate host.
The first documented human case of babesiosis was not recognized until about a half century later, when a splenectomized Croatian herdsman rapidly succumbed to an infection subsequently attributed to B. divergens.3 The first case in an immunocompetent person was identified on Nantucket Island, off the coast of Massachusetts, in 1969.4 The causative agent was B. microti,and the vector was the Ixodes dammini tick (now referred to as I. scapularis).5 Additional cases occurred on the island, and the disease became known as “Nantucket fever.” During the past decade, the incidence and geographic distribution of babesiosis have increased in the northeastern and upper midwestern regions of the United States. B. microti infection is almost as common as Lyme disease in some areas of southern New England, an observation that is consistent with the high prevalence of B. microti–infected ticks in the region.6-8 Babesiosis is now classified as a nationally notifiable disease and is recognized as an emerging health risk in several parts of the world.9,10
EPIDEMIOLOGY AND TRANSMISSION
More than 100 babesia species infect a wide array of wild and domestic animals, but only a few have been documented to infect humans.5,10-12 The overwhelming majority of cases in the United States are caused by B. microti. Such cases occur in the Northeast and upper Midwest, primarily from May through October (Figure 1
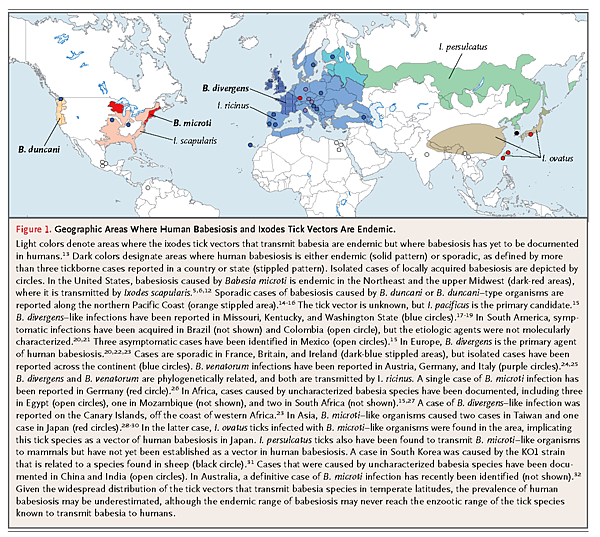
).10,12 The emergence of babesiosis in these regions has primarily been attributed to the expansion of the white-tailed deer population, encroachment of local communities on wildlife habitats, and greater awareness of the disease on the part of the public and physicians, although cases remain underreported.6,33-35 A small number of cases caused by B. duncani and B. duncani–type organisms have been identified on the Pacific Coast from northern California to Washington State.14-16 Sporadic cases of infection with B. divergens–like organisms have been documented in Kentucky, Missouri, and Washington State.17-19In Europe, most reported cases have been attributed to B. divergens, and a few have been caused by B. venatorum (formerly called EU1) and B. microti.20,22-26 In Asia, B. microti–like organisms have caused illness in Japan and Taiwan, whereas a new babesia agent (KO1 strain) has been identified in South Korea.28-31 Sporadic cases of babesiosis have been reported in Africa, Australia, and South America.15,20,21,27,32
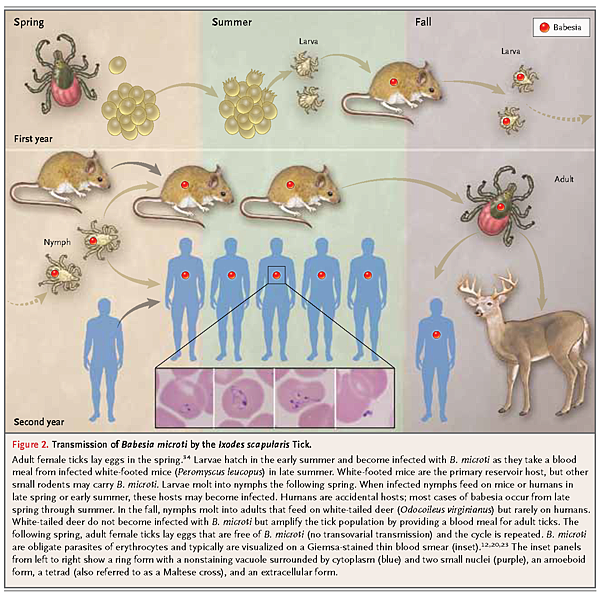
B. microti is primarily transmitted to people by I. scapularis.34 Progression of I. scapularis through each of the three stages of its life cycle (larva, nymph, and adult) requires a blood meal from a vertebrate host (Figure 2
Transmission ofBabesia microti by theIxodes scapularis Tick.
. The primary reservoir host for B. microti is the white-footed mouse (Peromyscus leucopus).34 Although adult ticks may transmit B. microti, most cases result from exposure to nymphal ticks during the period from late spring through summer.7,34 The tick vectors for transmission of B. duncani, B. duncani–type, and B. divergens–like parasites in the United States have yet to be conclusively identified.20 In Europe, the sheep tick I. ricinus is the primary vector for transmission of B. divergens and B. venatorum.20,23,36 Transplacental transmission of B. microti has been reported in a small number of cases.37
A few babesia species are transmitted through transfusion of blood or blood products.38-43 B. microti is the most common transfusion-transmitted pathogen reported to the Food and Drug Administration.38,41,44 More than 150 cases of transfusion-transmitted babesiosis have been identified since the first case was reported in 1979,45 and three quarters of these cases have occurred since 2000.46 Three cases have been caused by B. duncani.46,47 The actual number of cases caused by B. microti and B. duncani is thought to be much greater because many are undetected or underreported.41,43,46 Cases of transfusion-transmitted babesiosis are often severe, since recipients of blood products frequently are immunocompromised or have coexisting medical conditions, and approximately a fifth of cases have been fatal.46,48,49 Infections are reported throughout the year, but most occur from early summer through late fall.46,49 About 10% of cases occur in nonendemic areas because persons may become infected at endemic sites and subsequently donate blood in nonendemic areas or because units of contaminated blood are exported to nonendemic areas.41-43,46
PHYLOGENETIC CLASSIFICATION
Babesia species belong to the phylum Apicomplexa, which includes the protozoan parasites causing malaria, toxoplasmosis, and cryptosporidiosis.11 Babesia species that infect humans can be classified into four clades.16,23,50 The first clade consists of B. microti, small parasites (<3 μm) that form a species complex in which nearly all human isolates belong to one subclade.51,52 The second clade includes B. duncani and B. duncani–type organisms, small babesia that are phylogenetically distinct from B. microti and are related to babesia of dogs and wildlife in the western United States.15,16 The third clade includes B. divergens, a parasite of cattle, and B. venatorum, which infects roe deer.23,24,36 These species are small but phylogenetically related to the large babesia (≥3 μm). The fourth clade consists of large babesia that infect ungulates and includes the KO1 strain.23,31
LIFE CYCLE OF PATHOGEN
B. microti undergo developmental changes within the tick vector and the reservoir host.20,23,53When larval ticks feed on infected mice in late summer (Figure 2), gametocytes accumulate in the tick gut and differentiate into gametes. Gametes fuse to form zygotes that migrate across the gut epithelium into the hemolymph, where they mature into ookinetes. The ookinetes move to the salivary glands and become dormant sporoblasts.53 As nymphal ticks feed in the early summer of the following year, several thousand sporozoites are delivered into the vertebrate host. Sporozoites attach to erythrocytes by docking onto glycosaminoglycans and sialoglycoproteins.54,55 Once inside the erythrocytes, sporozoites mature into trophozoites, which eventually bud to form four merozoites. Egress of merozoites is accompanied by rupture of the host erythrocyte and invasion of other erythrocytes.
CLINICAL MANIFESTATIONS
The clinical manifestations of babesiosis range from subclinical infection to fulminating disease resulting in death.12 Most symptomatic patients become ill 1 to 4 weeks after the bite of a B. microti–infected tick and 1 to 9 weeks (but up to 6 months in one reported case) after transfusion of contaminated blood products.12,46 After a gradual onset of malaise and fatigue, fever usually develops, with a peak temperature that can be as high as 40.9°C (105.6°F). Chills and sweats are common and may be accompanied by headache, myalgia, anorexia, nonproductive cough, arthralgia, and nausea.6,56-61 Occasional symptoms include vomiting, sore throat, abdominal pain, conjunctival injection, photophobia, weight loss, emotional lability, depression, and hyperesthesia.12,56 On physical examination, fever is the most common sign. It may be accompanied by splenomegaly or occasionally by pharyngeal erythema, hepatomegaly, jaundice, or retinopathy with splinter hemorrhages and retinal infarcts.12,56,57
Laboratory findings that are consistent with a mild-to-moderate hemolytic anemia include a low hematocrit, low hemoglobin level, low haptoglobin level, elevated reticulocyte count, and elevated lactate dehydrogenase level.58,60,61 Thrombocytopenia is commonly observed. The illness usually lasts for 1 or 2 weeks, but fatigue may persist for months.56,57,59,62 Asymptomatic parasitemia may persist for several months after standard therapy is initiated or for more than a year if the patient does not receive treatment.56,62 Illness may relapse in severely immunocompromised patients despite 7 to 10 days of antimicrobial therapy and may persist for more than a year if not adequately treated.62-66
The severity of babesiosis depends primarily on the immune status of the patient and on the babesia species causing the infection. About half of children and a quarter of previously healthy adults who are infected with B. microti have no symptoms.6 Asymptomatic, mild, and moderate infections generally occur in people who are immunocompetent.6,12,33 In contrast, severe B. microtiillness requiring hospital admission is common among patients who have undergone splenectomy and those with cancer, human immunodeficiency virus infection, hemoglobinopathy, or chronic heart, lung, or liver disease.49,60,63-66 Other groups at increased risk for severe disease include neonates, persons over the age of 50 years, patients receiving treatment with immunosuppressive drugs for cancer (e.g., rituximab) or undergoing organ transplantation, and those receiving anticytokine therapy (e.g., etanercept and infliximab).25,37,58,60,65-69 Complications develop in approximately half of patients who are hospitalized with babesiosis. The acute respiratory distress syndrome and disseminated intravascular coagulopathy are the most common complications, but congestive heart failure, coma, liver failure, renal failure, or splenic rupture also may occur.12,58,60Fatality rates of 6 to 9% have been reported among hospitalized patients and up to 21% among those with immunosuppression.58,60,65 Cases caused by B. duncani and B. duncani–type organisms have ranged from asymptomatic to fatal.14,15,47 Most reported cases of B. divergensinfection are severe and occur in people who lack a spleen.20,22 The fatality rate for B. divergensinfection has dramatically declined since the introduction of aggressive treatment with a combination of antimicrobial agents and exchange transfusion.20,22 All five reported cases of B. divergens–like infection occurred in asplenic patients and were severe, resulting in the deaths of two patients.17-19,23
HOST RESISTANCE AND PATHOGENESIS
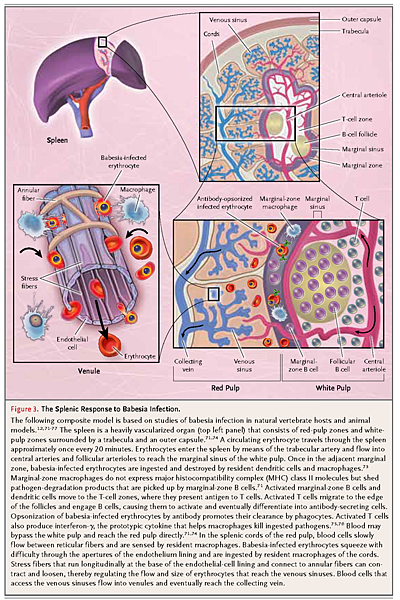
Our understanding of host resistance against babesia species that infect humans is limited and based on human case studies and studies of babesiosis in natural vertebrate hosts and animal models. Splenectomy is a major risk factor for severe infection, regardless of the babesia species.58,60,64-66,70 The spleen plays a central role in host defense by clearing infected erythrocytes from the bloodstream and mounting a protective immune response (Figure 3
The Splenic Response to Babesia Infection.).71-74
CD4+ T cells in mice with B. microti infection and natural killer cells in those with B. duncani infection produce interferon-γ, the prototypic type 1 helper T-cell (Th1) cytokine that promotes killing of intracellular pathogens by macrophages and enhances antibody production by B cells.75-77 B cells are critical for resolution of B. microti infection in patients whose cellular immunity is impaired and most likely help to clear B. microti in immunocompetent patients.25,65,69 The age-related decline in cellular immunity helps explain the severity of babesiosis in patients over the age of 50 years. In a mouse model of age-related susceptibility to B. microtiparasitemia, the protection conferred by adoptive transfer of splenic immune cells is age-dependent and genetically determined.78
The pathogenesis of babesiosis is closely linked to the host response to infection and parasite-induced modifications in the erythrocyte membrane. In mild cases of babesiosis, inflammatory cytokines (e.g., tumor necrosis factor α [TNF-α] and interleukin-6) and adhesion molecules (e.g., E-selectin, intracellular adhesion molecule 1 [ICAM-1], and vascular-cell adhesion molecule 1 [VCAM-1]) are up-regulated.29 Excessive synthesis of cytokines, however, may result in severe babesiosis and associated complications.72 Pulmonary inflammation in mice with B. duncani infection has been associated with excessive production of TNF-α and interferon-γ, and blockade of either cytokine prevents death.77,79,80 Intravascular sequestration of leukocytes and infected erythrocytes may lead to obstruction of the microvasculature and tissue hypoxia.72,81,82 Some babesia species export proteins to the surface of infected erythrocytes, resulting in the adherence of these erythrocytes to the vascular endothelium and in their delayed clearance by the spleen.82As with Plasmodium falciparum, the protein that is critical for cytoadherence of B. bovis–infected erythrocytes is encoded by a variable multicopy gene family that contributes to immune evasion.72,82 Cytoadherence of babesia-infected erythrocytes has yet to be documented in humans. Anemia that is caused by the rupture of erythrocytes during egress of babesia also contributes to pathogenesis, as do nonhemolytic mechanisms, such as the clearance of uninfected erythrocytes.83
DIAGNOSIS
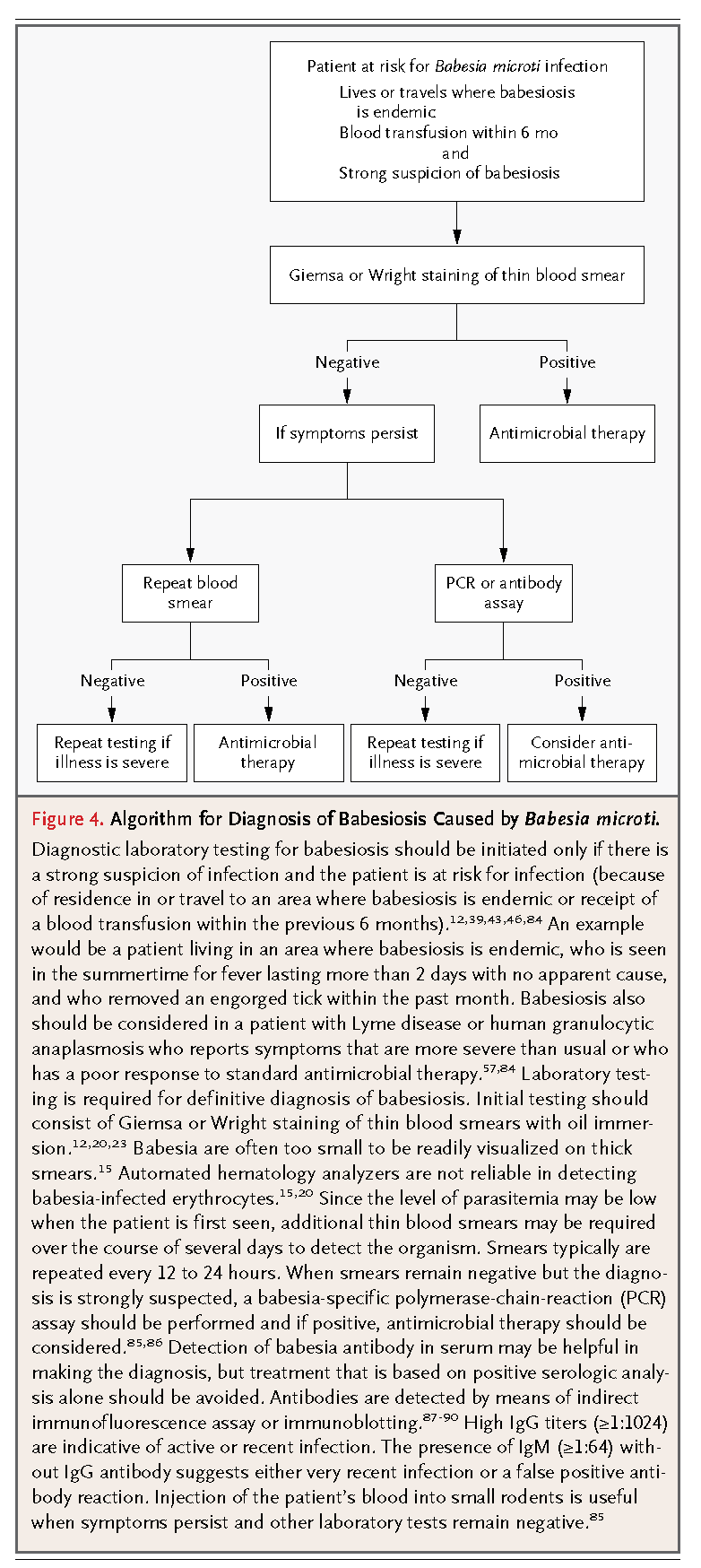
Babesiosis should be considered in any patient with an unexplained febrile illness who has resided in or traveled to an area where the infection is endemic within the previous 2 months or who has received a blood transfusion within the previous 6 months (Figure 4
Algorithm for Diagnosis of Babesiosis Caused by Babesia microti.).12,39,43,46,84
The diagnosis requires a strong clinical suspicion because of the lack of an easily recognized clinical sign, such as the erythema migrans rash of Lyme disease. Since I. scapularis ticks can transmit B. microti, Borrelia burgdorferi, and Anaplasma phagocytophilum, babesiosis should be suspected in patients in whom Lyme disease or anaplasmosis has been diagnosed if more severe disease develops or if they have a poor response to standard antimicrobial therapy.7,8,57,84,91
A definitive diagnosis is generally made by microscopical identification of babesia on thin blood smears with Giemsa or Wright staining (Figure 2).12,15,20,23 B. microti trophozoites appear as pleomorphic ring forms (round, oval, pear-shaped, or amoeboid) and are indistinguishable from B. duncani trophozoites. Although rare, tetrads of merozoites that are arranged in a cross-like pattern (a so-called Maltese cross) are pathognomonic for babesiosis caused by B. microti or B. duncani.12,16 B. divergens andB. venatorum merozoites typically appear as paired pear-shaped forms but also rarely appear as tetrads in human red cells.20,24 Although ring forms of babesia may resemble those of P. falciparum, malaria can be eliminated from consideration on the basis of a travel history and a careful review of blood smears.12,15,20 Distinguishing features of babesia are pleomorphic ring forms, extracellular forms, the absence of identifiable gametocytes, and the absence of brown deposits (hemozoin). The level of parasitemia is generally between 1 and 10% but can be as high as 80%.12,58,60,70 Because the parasitemia level is often less than 1% early in the course of illness, at least 300 microscopical fields should be reviewed (Figure 4).
Other laboratory tests are useful in establishing the diagnosis, especially when smears are negative. A polymerase-chain-reaction (PCR) assay is highly sensitive and specific for the detection of babesia DNA in blood, particularly with real-time technology.85,86 The standard assay for the detection of babesia antibody is the indirect immunofluorescence assay.87-89 IgM antibody usually is first detected 2 weeks after the onset of illness.89 IgG titers often exceed 1:1024 during the acute phase of illness and decline to 1:64 or less within 8 to 12 months.62,87 An immunoblot assay for detection of B. microti antibody is also available.90 Assays for B. microti antibody do not detect B. duncani, B. divergens, or B. venatorum antibody.14,15,20,43 When laboratory tests are inconclusive and infection is strongly suspected, a blood sample from the patient can be injected into a laboratory animal, such as a hamster. B. microti organisms usually appear in the blood of the inoculated animal within 2 to 4 weeks.85
TREATMENT AND OUTCOME
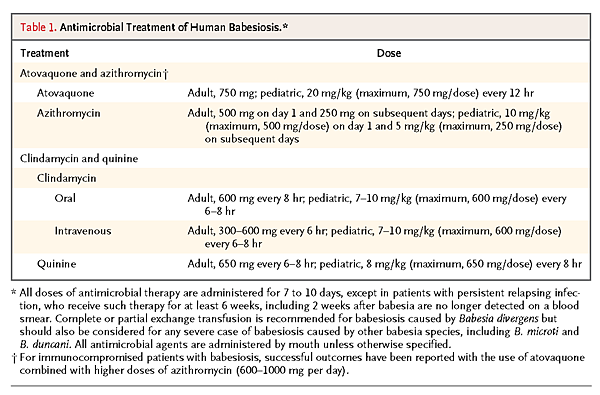
A combination of atovaquone and azithromycin is the treatment of choice for immunocompetent patients with mild-to-moderate babesiosis (Table 1
Antimicrobial Treatment of Human Babesiosis.).59,92
One study showed that this combination was as effective as clindamycin and quinine in clearing parasitemia and resolving symptoms.59 Only 15% of 41 patients receiving atovaquone and azithromycin had symptoms consistent with an adverse drug reaction, and only 1 patient (2%) had to discontinue the medications because of side effects. In contrast, three fourths of 18 patients receiving clindamycin and quinine had adverse drug reactions, and dose reduction or discontinuation of treatment was required in a third of these patients. Even patients with mild babesiosis should be treated with atovaquone and azithromycin because without treatment, the infection may not be cleared and severe disease may develop or the patients may inadvertently transmit the infection by donating blood. Treatment of asymptomatic carriers should be considered if parasites are detected for longer than 3 months.92
The combination of clindamycin and quinine was the first successful antimicrobial regimen for the treatment of B. microti infection,93 and intravenous clindamycin and oral quinine are still recommended for patients with severe babesial illness (Table 1).92 When necessary, intravenous quinidine can be used instead of oral quinine but requires cardiac monitoring for possible prolongation of the QT interval. Partial or complete exchange transfusion of whole blood or packed red cells should be considered in patients with severe disease, particularly those infected with B. divergens.12,20,23,45,94 Indications for such therapy include a high level of parasitemia (≥10%), clinically significant anemia, or renal, hepatic, or pulmonary compromise.12,60,64,92
Severely immunocompromised patients may have persistent or relapsing babesiosis despite treatment with the standard course of 7 to 10 days of antimicrobial agents.65 In such patients, a cure generally requires at least 6 weeks of antimicrobial therapy, including 2 weeks after babesia are no longer detected on blood smears.65 High doses of azithromycin in combination with atovaquone have been used successfully in immunocompromised patients (Table 1).95 Resistance to atovaquone and azithromycin occasionally develops during prolonged therapy (>4 weeks) that follows an initial, subcurative course of this combination.66
Patients with babesiosis should be closely monitored during therapy. In most cases, symptoms abate within a day or two after antimicrobial therapy is initiated, and infection resolves within 3 months.59,62,92 In severely ill patients, parasitemia should be monitored daily until it has decreased to a level of less than 5% and the patient's condition has improved. If symptoms recur, treatment should immediately resume, with close clinical follow-up.
PREVENTION
Preventive measures consist of personal, residential, and community approaches.35,96-99 Personal protective measures include avoiding sites where ticks, mice, and deer thrive. It is especially important for persons at increased risk, such as asplenic or other immunocompromised persons who live in or travel to areas where babesiosis is endemic, to avoid deciduous forests and the edge between woodlands and open areas, where ticks may abound.35,96,97 Persons who cannot avoid such areas should wear protective clothing, apply tick repellents containing permethrin or N,N-diethyl-meta-toluamide (DEET) to clothing and repellents containing DEET to skin, and examine themselves daily for ticks.98 Landscape-management strategies, such as keeping grass mowed, removing leaf litter, using plantings that do not attract deer, and spraying areas of high tick density with acaricidal formulations, may help reduce the risk of tickborne infections.97,99 The elimination of deer populations sharply reduces the risk of infection but is difficult to implement.35 Public education about the risks and characteristic symptoms of tickborne diseases is an important part of these preventive measures.
The current approach of questioning prospective blood donors about a history of babesiosis and indefinitely deferring those who have had the disease has not been effective in preventing transfusion-transmitted babesiosis.39,41,43 Laboratory-based screening programs to identify prospective blood donors who are infected with babesia are being developed for use in areas where babesiosis is endemic. An interim analysis of the first such program showed that the combined use of an indirect immunofluorescence assay to detect B. microti antibody and a real-time PCR assay to detect B. microti DNA reduced the incidence of transfusion-transmitted babesiosis in neonates and children with sickle cell disease or thalassemia.100 On the basis of the recent emergence of tickborne and transfusion-transmitted babesiosis, the development of a vaccine against human babesiosis should be considered.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 