快樂小藥師
目前分類:FDA 公告 (120)
- Jun 13 Mon 2011 08:53
[FDA Review Concludes] No Cancer Risk With ARBs
An FDA review launched in the wake of a controversial 2010 meta-analysis by DrIlke Sipahi (University Hospitals Case Medical Center, Cleveland, OH) et al [1] suggesting an increased risk of cancer among patients taking angiotensin-receptor blockers (ARBs) has concluded that the drugs do not pose a cancer risk to patients [2]. But reacting to the FDA alert to heartwire , Sipahi said he was disappointed with how the FDA conducted its review and, in particular, its failure to do a patient-level analysis.
- Jun 10 Fri 2011 05:44
[Drug Safety and Availability]Questions and Answers:5-alpha reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer
5-alpha reductase inhibitors(5-alpha 還原酶抑制劑)有可能會增加罹患前列腺癌的風險嗎?
On June 9, 2011, the U.S. Food and Drug Administration (FDA) informed the public of new safety information for drugs called 5-alpha reductase inhibitors (5-ARIs). Men who take these drugs may have an increased risk of being diagnosed with a more serious form of prostate cancer (high-grade prostate cancer). The Warnings and Precautions section of the labels for all FDA-approved 5-ARIs have been revised to include information about this risk.
- Jun 09 Thu 2011 08:48
[FDA Approves] Once-Daily Rilpivirine for HIV Infection

The US Food and Drug Administration (FDA) announced today the approval of once-daily rilpivirine (Edurant, Tibotec Therapeutics, a division of Centocor Ortho Biotech Inc) for treatment of HIV infection. Rilpivirine is a nonnucleoside reverse transcriptase inhibitor (NNRTI) that may be taken once daily in combination with other antiretroviral drugs for HIV-infected, treatment-naive patients.
- May 04 Wed 2011 08:58
FDA Approves New Drug (linagliptin) for Type 2 Diabetes
The US Food and Drug Administration (FDA) today approved linagliptin (Tradjenta, Eli Lilly Co and Boehringer Ingelheim Pharmaceuticals) for improving blood glucose control in adults with type 2 diabetes, either as a stand-alone or in combination with other therapies.
- Apr 30 Sat 2011 06:15
FDA Drug Safety Communication: Safety update on Progressive Multifocal Leukoencephalopathy (PML) associated with Tysabri (natalizumab)
- Apr 24 Sun 2011 05:28
[FDA Approvals]Fidaxomicin Noninferior to Vancomycin for Treatment of C Difficile Infection

Compared with vancomycin, fidaxomicin (Optimer Pharmaceuticals, Inc) produces noninferior rates of clinical cure in adults with Clostridium difficile infection. In addition, for some strains, fidaxomicin is associated with a significantly lower rate of recurrence, according to the findings of a randomized trial.
- Apr 22 Fri 2011 05:38
[PDR Drug Alert]Serious Risks of QT prolongation, Torsades de pointes and Sudden death for vandetanib; FDA required restricted distribution program.
- Apr 10 Sun 2011 05:25
[FDA Medwatch]Benzocaine Topical Products: Sprays, Gels and Liquids - Risk of Methemoglobinemia(變性血紅素血症)
- Mar 31 Thu 2011 05:59
FDA Drug Safety Communication: Special storage and handling requirements must be followed for Pradaxa (dabigatran etexilate mesylate) capsules
The U.S. Food and Drug Administration (FDA) is alerting the public to important storage and handling requirements for Pradaxa (dabigatran etexilate mesylate) capsules. Due to the potential for product breakdown from moisture and loss of potency, Pradaxa capsules should only be dispensed and stored in the original bottle or blister package and patients should be aware of the specific handling requirements.
- Mar 26 Sat 2011 05:10
FDA Approves Ipilimumab for Advanced Melanoma
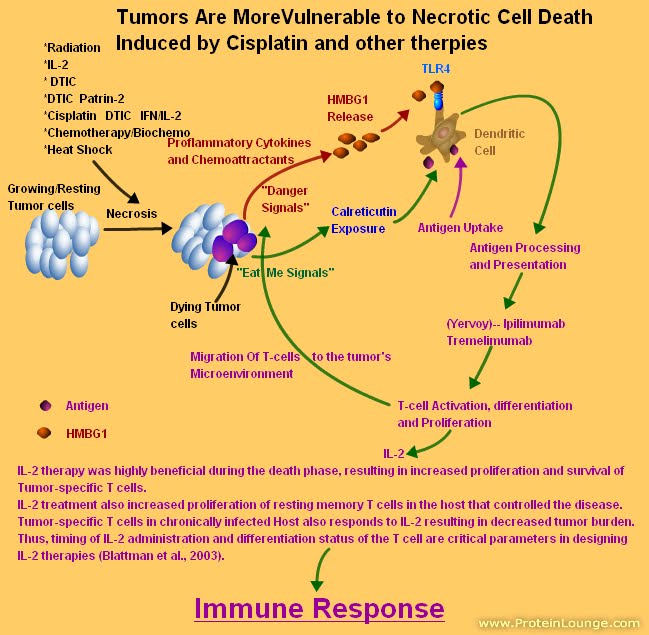
The US Food and Drug Administration (FDA) announced today the approval of ipilimumab (Yervoy, Bristol-Myers Squibb) for the treatment of advanced melanoma as a second-line therapy.
- Mar 05 Sat 2011 07:03
FDA Approves COPD Drug Opposed by Advisory Panel

The US Food and Drug Administration (FDA) announced today that it has approved a new, once-a-day drug to treat chronic obstructive pulmonary disease (COPD), even though an agency advisory panel of outside experts had opposed it on safety and efficacy grounds.
- Mar 04 Fri 2011 06:28
[Enforcement Activities by FDA]Unapproved Prescription Cough, Cold, and Allergy Products
美國食品暨藥物管理局(FDA)今天宣布,將把500多種可能有安全疑慮的感冒、咳嗽和過敏處方藥下架。
- Feb 20 Sun 2011 06:53
FDA Warns Against Use of Terbutaline to Treat Preterm Labor
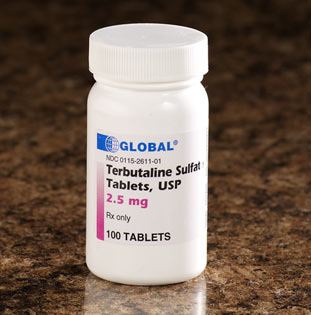
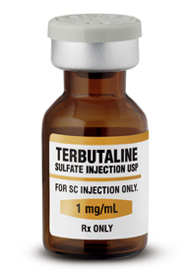
Clinicians should not use injectable terbutaline to prevent preterm labor or treat it beyond 48 to 72 hours because of the risk for maternal heart problems and death, the US Food and Drug Administration (FDA) announced today.
- Feb 09 Wed 2011 06:49
[FDA Drug Safety] Communication: Avandia (rosiglitazone) labels now contain updated information about cardiovascular risks and use in certain patients
- Dec 25 Sat 2010 09:47
FDA Drug Safety Communication: Ongoing safety review of Recombinant Human Growth Hormone (somatropin) and possible increased risk of death
- Dec 16 Thu 2010 06:14
FDA Drug Safety Communication: Death resulting from overdose after accidental ingestion of Tessalon (benzonatate) by children under 10 years of age
這個藥物在台灣有些醫院或是診所也很常用,就是bensau。
[12-14-2010] The U.S. Food and Drug Administration (FDA) is warning the public that accidental ingestion of benzonatate by children under the age of 10 years can result in death from overdose.
The U.S. Food and Drug Administration is alerting drug and dietary supplement manufacturers, compounding pharmacies, and distributors of Povidone analogs (Povidone/Copovidone/Crospovidone), that the agency recently detected excessive levels of peroxide in one lot of Crospovidone (cross linked polyvinyl N-pyrrolidone) manufactured by China-based Tianjin Boai NKY International Ltd1. The peroxide level found by the FDA in the lot was more than four (4) times the maximum level of peroxide (400 ppm) allowed by global compendial monographs.





 線上藥物查詢
線上藥物查詢 