
The US Food and Drug Administration (FDA) today approved bosutinib (Bosulif, Pfizer) as a new treatment option for adult patients with previously treated Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML).

The US Food and Drug Administration (FDA) today approved bosutinib (Bosulif, Pfizer) as a new treatment option for adult patients with previously treated Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML).

The US Food and Drug Administration (FDA) has approved a second oral agent for the treatment of multiple sclerosis (MS), teriflunomide (Aubagio, Genzyme/Sanofi).
Lopinavir/ritonavir oral solution (Kaletra, Abbott Laboratories) should be avoided in premature or full-term infants for the first 14 days after their due dates because of possible cardiac, renal, or respiratory problems, the US Food and Drug Administration (FDA) announced today in a safety alert announcing a label change.

The US Food and Drug Administration today approved hydroxyprogesterone caproate injection (Makena) to reduce the risk for preterm delivery before 37 weeks of pregnancy in women with singleton pregnancy and a history of at least 1 spontaneous preterm birth.
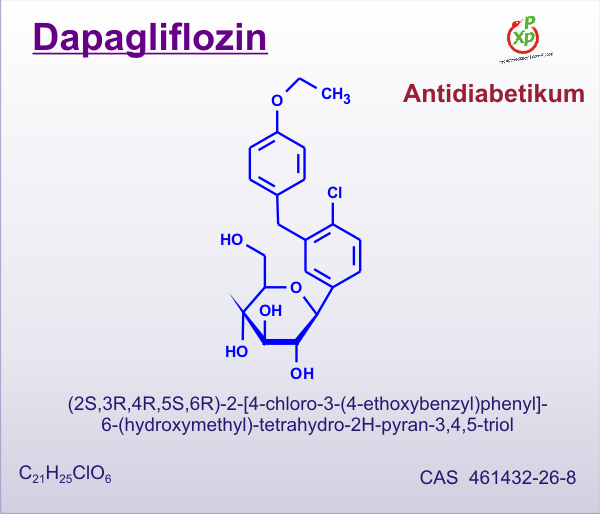
The US Food and Drug Administration (FDA) has declined approval of the novel diabetes drug dapagliflozin, asking codevelopers Bristol-Myers Squibb and AstraZeneca to provide more data about the drug's risk–benefit profile.

Baxter International Inc. today announced that the U.S. Food and Drug Administration (FDA) has approved ADVATE [Antihemophilic Factor (Recombinant) Plasma/Albumin Free Method] for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in patients with hemophilia A. ADVATE is the only antihemophilic factor approved in the United States for prophylactic use in both adults and children.
FDA核准 Jakafi 治療骨髓纖維化的藥物
The U.S. Food and Drug Administration today approved Jakafi (ruxolitinib), the first drug approved to specifically treat patients with the bone marrow disease myelofibrosis.

The US Food and Drug Administration (FDA) today approved tadalafil (Cialis, Eli Lilly), a phosphodiesterase-5 inhibitor, to also treat the signs and symptoms of benign prostatic hyperplasia (BPH) as well as a combination of BPH and erectile dysfunction (ED) when the conditions coincide.
On September 26, 2011, FDA informed the public that it has not yet reached a conclusion, but remains concerned, about the potential increased risk of blood clots with the use of drospirenone-containing birth control pills. FDA has completed its review of the two 2011 studies that looked at the risk of blood clots for women who use drospirenone-containing birth control pills, previously mentioned in FDA's Drug Safety Communication issued on May 31, 2011 .1,2 FDA is continuing its review of a separate FDA-funded study that evaluated the risk of blood clots in users of several different hormonal birth control products (contraceptives). Preliminary results of the FDA-funded study suggest an approximately 1.5-fold increase in the risk of blood clots for women who use drospirenone-containing birth control pills compared to users of other hormonal contraceptives.

The U.S. Food and Drug Administration (FDA) is warning the public that serious allergic reactions have been reported with the use of the antipsychotic medication Saphris (asenapine maleate). TheContraindications, Warnings and Precautions, Adverse Reactions, and Patient Counseling Informationsections of the Saphris drug label have been revised to include information about this risk and to inform healthcare professionals that Saphris should not be used in patients with a known hypersensitivity to the drug.
The US Food and Drug Administration has granted accelerated approval of brentuximab vedotin infusion (Adcetris, Seattle Genetics, Inc) for the treatment of relapsed or refractory Hodgkin's lymphoma and systemic anaplastic large cell lymphoma.
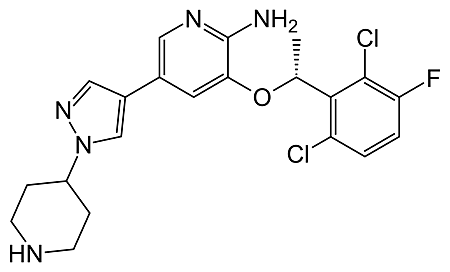
The US Food and Drug Administration (FDA) has approved crizotinib (Xalkori, Pfizer) for the treatment of late-stage non-small cell lung cancer (NSCLC) in patients who express the abnormal anaplastic lymphoma kinase (ALK) gene.
The US Food and Drug Administration (FDA) has approved nitroglycerin ointment 0.4% (Rectiv, ProStrakan Group) for the treatment of moderate to severe pain associated with chronic anal fissures, the company announced today.
The US Food and Drug Administration (FDA) late yesterday approved the first autologous aesthetic cell therapy to improve the appearance of moderate to severe nasolabial fold wrinkles in adults.

The US Food and Drug Administration (FDA) approved ezogabine (Potiga, Valeant Pharmaceuticals and GlaxoSmithKline [GSK]) as an add-on medication to treat seizures associated with epilepsy in adults.

The US Food and Drug Administration (FDA) today approved a sterile, injectable gel called Solesta (Oceana Therapeutics) to treat fecal incontinence in patients after other remedies have failed.