Dynastat:COX-2的針劑
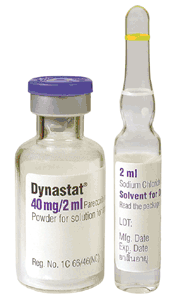
包裝:Dynastat 20 mg powder for solution for injection
學名:Parecoxib (valdecoxib (its active metabolite))
結構:
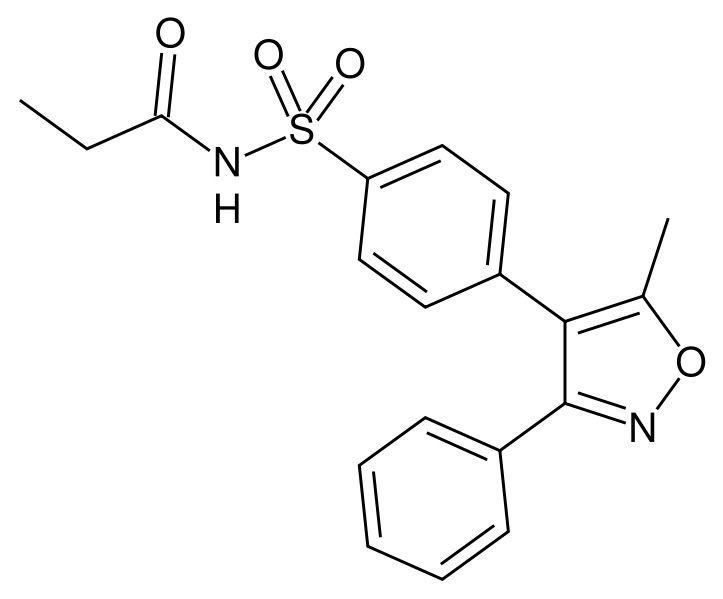
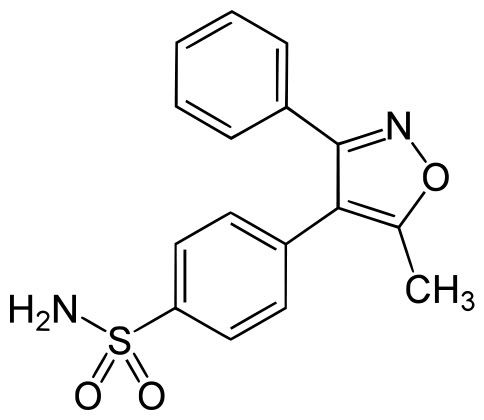
活性代謝物valdecoxib結構:
QUALITATIVE AND QUANTITATIVE COMPOSITION
20 mg vial: Each vial contains 20 mg parecoxib (present as 21.18 mg parecoxib sodium) for reconstitution. After reconstitution, the final concentration of parecoxib is 20 mg/ml.
When reconstituted in sodium chloride 9 mg/ml (0.9%) solution, Dynastat contains approximately 0.22 mEq of sodium per vial.
治療適應症:Therapeutic indications
For the short-term treatment of postoperative pain.
給藥方式
The recommended dose is 40 mg administered intravenously (IV) or intramuscularly (IM), followed every 6 to 12 hours by 20 mg or 40 mg as required, not to exceed 80 mg/day.
以靜脈注射或是肌肉注射方式給予,每6-12好時給予20~40 mg,每天不要超過80mg。
The IV bolus injection may be given rapidly and directly into a vein or into an existing IV line.
可以是用快速靜脈推注
The IM injection should be given slowly and deeply into the muscle.
肌肉注射請採用深層慢速給予。
Precipitation may occur when Dynastat is combined in solution with other medicinal products and therefore Dynastat must not be mixed with any other drug, either during reconstitution or injection.
在配製或是注射時,請勿與其他藥物混合。
In those patients where the same IV line is to be used to inject another medicinal product, the line must be adequately flushed prior to and after Dynastat injection with a solution of known compatibility.
如果不得以需要使用同一條管路時,請在使用前後進行清洗。
配製方式:
Reconstitution process Use aseptic technique to reconstitute lyophilised parecoxib (as parecoxib sodium).
Remove the yellow flip-off cap to expose the central portion of the rubber stopper of the 20 mg parecoxib vial. Withdraw, with a sterile needle and syringe, 1 ml of an acceptable solvent and insert the needle through the central portion of the rubber stopper transferring the solvent into the 20 mg vial. Dissolve the powder completely using a gentle swirling motion and inspect the reconstituted product before use. The entire contents of the vial should be withdrawn for a single administration.
After reconstitution, Dynastat should be inspected visually for particulate matter and discoloration prior to administration. The solution should not be used if discolored or cloudy, or if particulate matter is observed. Dynastat should be administered within 24 hours of reconstitution, or discarded.
IV line solution compatibility
After reconstitution with acceptable solvents, Dynastat may only be injected IV or IM, or into IV lines delivering:
sodium chloride 9 mg/ml (0.9%) solution
glucose 50 g/l (5%) solution for infusion
sodium chloride 4.5 mg/ml (0.45%) and glucose 50 g/l (5%) solution for injection
Ringer-Lactate solution for injection
Injection into an IV line delivering glucose 50 g/l (5%) in Ringer-Lactate solution for injection, or other IV fluids not listed above, is not recommended as this may cause precipitation from solution.
肝腎功能劑量調整:
Hepatic Impairment: No dosage adjustment is generally necessary in patients with mild hepatic impairment (Child-Pugh score 5-6). Introduce Dynastat with caution and at half the usual recommended dose in patients with moderate hepatic impairment (Child-Pugh score 7-9) and reduce the maximum daily dose to 40 mg. There is no clinical experience in patients with severe hepatic impairment (Child-Pugh score ≥10), therefore its use is contraindicated in these patients.
Renal Impairment: On the basis of pharmacokinetics, no dosage adjustment is necessary in patients with mild to moderate (creatinine clearance of 30-80 ml/min.) or severe (creatinine clearance < 30 ml/min.) renal impairment. However, caution should be observed in patients with renal impairment or patients who may be predisposed to fluid retention.
Symptoms of an allergic reaction to these medicines may include:
* asthma, wheezing or shortness of breath
* swelling of the face, lips or tongue which may cause difficulty in swallowing or
breathing
* hives, itching or skin rash
* swelling, blistering or peeling of the skin.
These symptoms may be severe if you are allergic to sulfonamides or to any of the ingredients listed.
如果有以下情況,請勿使用本針劑:
Do not use DYNASTAT if:
* you are about to undergo heart or blood vessel surgery
準備要進行心臟血管手術
* you have heart disease or have had a heart attack or stroke
你有心臟疾患或是中風
* Severe hepatic dysfunction (serum albumin <25 g/l or Child-Pugh score ≥ 10).
有嚴重肝疾病(serum albumin <25 g/l or Child-Pugh score ≥ 10)
Active peptic ulceration or gastrointestinal (GI) bleeding.
正罹患腸胃道潰瘍的患者。
Congestive heart failure (NYHA II-IV).
鬱血性心衰竭患者(NYHA II-IV)
The third trimester of pregnancy and breast-feeding
懷孕第三期及授乳婦女
補充一點,其實在2005年就已經有一則新聞提過這個藥物了:
Dynastat Rejected by FDA
FDA rejects Pfizer’s application to sell injectable COX-2 inhibitor Dynastat in U.S. - 9/20/05
The U.S. Food and Drug Administration issued a nonapprovable letter for parecoxib to Pfizer, Inc., a drug that is sold in the European Union and other foreign markets under the brand name Dynastat. The drug is a "chemical cousin" of the COX-2 inhibitor Bextra, which was recently removed from the U.S. market after the FDA said the risks associated with taking Bextra outweighed its possible benefits.
Dynastat is prescribed in the E.U. and other foreign countries to treat acute pain for surgery patients and is the only liquid form of a COX-2 inhibitor. Other COX-2 inhibiting drugs include Vioxx, which was withdrawn from the market last September, and Celebrex. COX-2 inhibitors have been linked to heart attacks, strokes, and other cardiovascular events in patients taking them for pain relief. Vioxx was withdrawn specifically after clinical trials showed that patients who took the drug for more than 18 months had a higher cardiovascular risk of adverse events than patients receiving a placebo.
Bextra was pulled from the market in April 2005 at the FDA's request, but Pfizer is in talks with the agency about returning the drug to market. Other companies are working on new COX-2 drugs, including Merck and Co., which gained conditional approval from the FDA recently for its drug Arcoxia, also a COX-2 cousin of Bextra and Vioxx. The FDA has asked for more safety and efficacy data before it will approve the drug for sale.
European Union regulators have said they continue to permit sales of Dynastat because it is injectable and it only used for a short time for pain relief after surgery.
Source: Robert Steyer, "Pfizer drug rejected," TheStreet.com, September 20, 2005





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 