NEJM:Volume 355:2444-2451
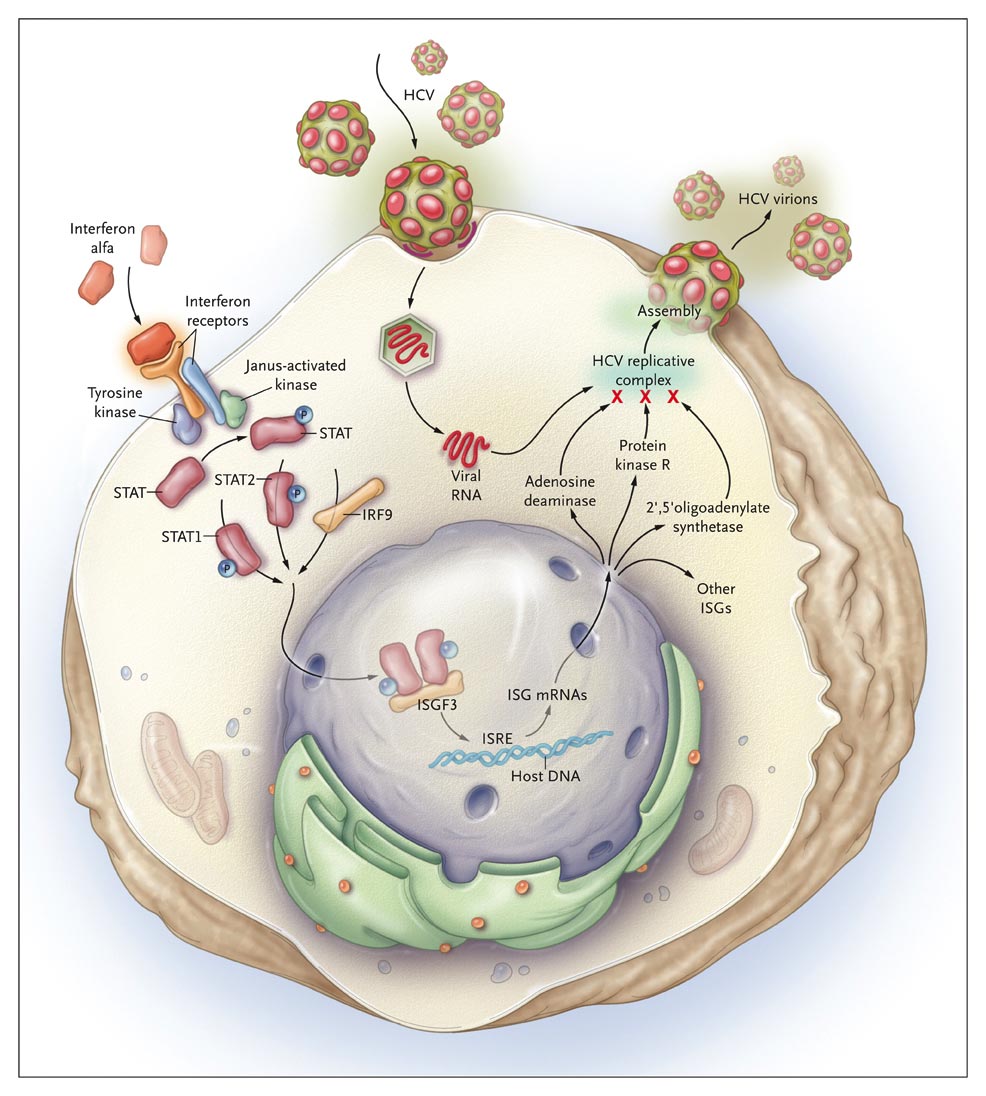
Interferon alfa engages receptors on the hepatocyte cell-surface membrane, causing them to dimerize and to activate Janus-activated and tyrosine kinases that phosporylate the cytoplasmic signal transducers and activators of transcription (STAT) proteins. STAT1 and STAT2 dimerize and bind interferon regulatory factor 9 (IRF9), creating a large complex (interferon-stimulated gene factor 3, or ISGF3) that is translocated into the nucleus, where it binds to interferon-stimulated response elements (ISREs) on DNA. This engagement causes transcription of multiple (>100) interferon-stimulated gene (ISG) mRNAs, which exit the nucleus and encode proteins that alter cell metabolism and interfere with virus replication, protein synthesis, and assembly. Major ISGs thought to be important in inhibiting HCV replication include 2',5' oligoadenylate synthetase, which activates antiviral RNases; RNA-specific adenosine deaminase, which edits viral RNA; and protein kinase R, which inactivates protein translation from viral mRNA. The HCV replicative complex is associated with the cytoplasmic membranes of hepatocytes and comprises RNA replicative intermediates, viral mRNA, structural and nonstructural viral proteins, and assembling virions.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 