
David J. Kuter, M.D., D.Phil., Mathias Rummel, M.D., Ralph Boccia, M.D., B. Gail Macik, M.D., Ingrid Pabinger, M.D., Dominik Selleslag, M.D., Francesco Rodeghiero, M.D., Beng H. Chong, M.D., Xuena Wang, Ph.D., and Dietmar P. Berger, M.D., Ph.D.
如果這個月都有看我的網誌,對這個藥就不陌生了:
[Drugs]Current and Future Management Options for Myelodysplastic Syndromes 骨髓發育不全症候群的治療與展望
Eltrombopag for the Treatment of Chronic Idiopathic Thrombocytopenic Purpura
紫斑症 Idiopathic Thrombocytopenic Purpura
Immune thrombocytopenia is an autoimmune disease characterized by low platelet counts due to both increased platelet destruction and suboptimal platelet production.1 After initial treatment with glucocorticoids or intravenous immune globulin or anti-D immune globulin, most adult patients require second-line medical therapy (e.g., azathioprine or rituximab) or surgical therapy (i.e., splenectomy).2 However, most first- and second-line medical treatments are short-acting, have severe side effects, or are potentially toxic.2-4 These problems can adversely affect the health and quality of life of patients.
Splenectomy is used to remove the major site of platelet destruction and increase the platelet count.5 In approximately two thirds of patients, the response to splenectomy lasts for at least 5 years, and additional therapy is not needed.6,7 Unfortunately, splenectomy is associated with significant and occasionally fatal perioperative and postoperative complications such as bleeding, infection, and thrombosis.8 Additional long-term complications include increased susceptibility to infection,9-11 thrombosis,12 an increase in deaths from cardiovascular disease by a factor of 2,13and an increased rate of pulmonary hypertension.12,14 In some patients, splenectomy may be contraindicated, owing to coexisting medical conditions.2
Thrombopoietin mimetics increase platelet counts in most patients with immune thrombocytopenia and reduce the risk of bleeding.15-17 Romiplostim is a thrombopoietin mimetic “peptibody” (antibody heavy chain containing a therapeutic peptide) comprising a human immunoglobulin IgG1 Fc domain covalently linked at each of its two C-terminals to two 14-amino-acid peptides that bind to and stimulate the thrombopoietin receptor. Continuous treatment with romiplostim increases platelet counts in patients with immune thrombocytopenia for up to 5 years, with few adverse effects.18,19Romiplostim may offer the potential for long-term effective treatment in patients who wish either to avoid or defer splenectomy or in whom splenectomy is contraindicated.
We aimed to evaluate the incidences of platelet response (a platelet count >50×109 per liter), treatment failure, and splenectomy and to ascertain the safety of 52 weeks of treatment with romiplostim or standard of care in patients with immune thrombocytopenia.
METHODS
Study Design
Our study was a multicenter, randomized, controlled, 52-week, open-label evaluation of the efficacy and side-effect profile of romiplostim as compared with medical therapies that are the standard of care for adult patients with immune thrombocytopenia who have not undergone splenectomy. The study was conducted according to the trial protocol (available with the full text of this article at NEJM.org). After completing the 52-week treatment period, patients who did not enter another romiplostim study began a 6-month post-treatment safety monitoring period. The institutional review board at each center approved the protocol, and all patients provided written informed consent.
In collaboration with the academic investigators, Amgen (the sponsor) designed the study, gathered the data, conducted the statistical analyses, and interpreted the results. All authors participated in the design or execution of the study, contributed to interpretation of the data, approved the final draft of the manuscript, and made the decision to submit it for publication. The authors had unrestricted access to the primary data and were not limited by Amgen in the writing of this article. The principal (academic) investigator wrote the initial draft of the manuscript; subsequently, professional writing assistance was provided through Amgen. The academic authors vouch for the accuracy and completeness of the reported data and analyses.
Patients
Adult patients who had immune thrombocytopenia (according to American Society of Hematology guidelines; see the Methods in the Supplementary Appendix, available at NEJM.org)20 and who had not undergone splenectomy were enrolled at investigational sites in North America, Europe, and Australia. Other eligibility criteria were a history of one or more types of therapy for immune thrombocytopenia and a pretreatment platelet count of less than 50×109 per liter. Examination of a bone marrow–biopsy specimen was required, to confirm the diagnosis of immune thrombocytopenia, for patients older than 60 years of age.
Key exclusion criteria were previous splenectomy, active cancer or stem-cell disorder, history of cancer, previous exposure to a thrombopoietin mimetic, pregnancy, and lactation. At the time of enrollment, patients could have been receiving any therapy for immune thrombocytopenia except experimental treatments.
Study Treatments
Patients were randomly assigned, in a 2:1 ratio, to receive romiplostim or the medical standard of care and were stratified on the basis of geographic region. In the romiplostim group, to achieve a target platelet count of 50×109 to 200×109 per liter, romiplostim was administered weekly at a starting dose of 3 μg per kilogram of body weight, which was increased to a maximum dose of 10 μg per kilogram, according to a dosing algorithm (Table 1 in the Supplementary Appendix). If the platelet count was 20×109 per liter or less for 4 consecutive weeks while the patient was receiving a stable romiplostim dose of 10 μg per kilogram, romiplostim treatment was discontinued.
All patients returned to the investigational site for protocol-required visits weekly through week 8. After week 8, patients returned to the investigational site at least every 4 weeks or as necessary to assess and adjust the study medications (romiplostim or standard-of-care therapy). The quality of life was assessed at baseline and every 12 weeks thereafter until the end of treatment.
Treatments for patients assigned to receive the standard of care were selected by the investigator on the basis of standard institutional practices or therapeutic guidelines. Throughout the study, patients in either treatment group could receive additional therapies for immune thrombocytopenia (including short-term rescue therapies such as intravenous immune globulin, but excluding other thrombopoietin mimetics and investigational products) if they were deemed medically necessary by the investigator.
If therapy in either group was considered to be ineffective or associated with severe side effects, investigators could perform a splenectomy. Patients in whom splenectomy failed to increase the platelet count could be treated with either romiplostim or the standard of care, at the investigator's discretion; for such patients, only data from the time in the randomly assigned treatment group were included in the final analysis.
Outcome Measures and Follow-up Visits
There were two coprimary end points: the incidence of treatment failure and the incidence of splenectomy. Treatment failure was defined as a platelet count of 20×109 per liter or lower for 4 consecutive weeks at the highest recommended dose, a major bleeding event, or requirement for a change in therapy (including splenectomy) because of an adverse event or bleeding symptoms. Given the length of the treatment period, it was anticipated that some patients might discontinue the study early; therefore, in the primary end point analyses, a patient who had received any study treatment and had then discontinued the study was counted as having had both treatment failure and splenectomy.
Secondary efficacy end points included the time to splenectomy, platelet count, platelet response (platelet count >50×109 per liter at any scheduled visit, excluding counts obtained after discontinuation of the randomized treatment or within 8 weeks after receipt of rescue medications), and quality of life. The quality of life was assessed by means of the Immune Thrombocytopenic Purpura Patient Assessment Questionnaire (ITP-PAQ), consisting of 44 items specific to immune thrombocytopenia on each of 10 scales (with scores on each ranging from 0 to 100 and higher scores indicating a better quality of life).21
Safety end points included bleeding events, blood-product transfusions, and laboratory results. Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 3.0.
Statistical Analysis
The intention-to-treat patient population, which included all randomized patients, was used in analyses of baseline characteristics and all primary and secondary end points. Analyses of safety end points included patients who received at least one dose of study medication and were based on the actual treatment each patient received. A target sample size of approximately 210 patients, randomly assigned to either romiplostim or standard-of-care therapy in a 2:1 ratio, was estimated to provide over 99% power to detect a difference between the two groups in the incidence of treatment failure and approximately 90% power to detect a between-group difference in the incidence of splenectomy (with the use of a one-sided chi-square test at a significance level of 0.025).
Baseline characteristics and laboratory results were summarized for the two groups by means of descriptive statistics. The Cochran–Mantel–Haenszel test (with control for the geographic location of investigational sites) was used to analyze both coprimary end points. The Kaplan–Meier method and the log-rank test were used to evaluate between-group differences in the time to splenectomy.
To account for differences in the duration of receipt of study medication between the two treatment groups, incidence rates per 100 patient-weeks were calculated for adverse events, after adjustment for duration of exposure to the study medication. P values for ad hoc analyses of between-group differences in bleeding events were obtained with the use of Poisson regression models.
The two treatment groups did not differ significantly with respect to any baseline characteristics

The median age across the two groups was 57 years (with 36% of patients >65 years of age and 18% >75 years). The median duration of treatment for immune thrombocytopenia at the time of study entry was approximately 2 years, but 36% of patients (85 of 234) entered the study after having immune thrombocytopenia for a year or less (median duration in this subgroup, 0.25 years).
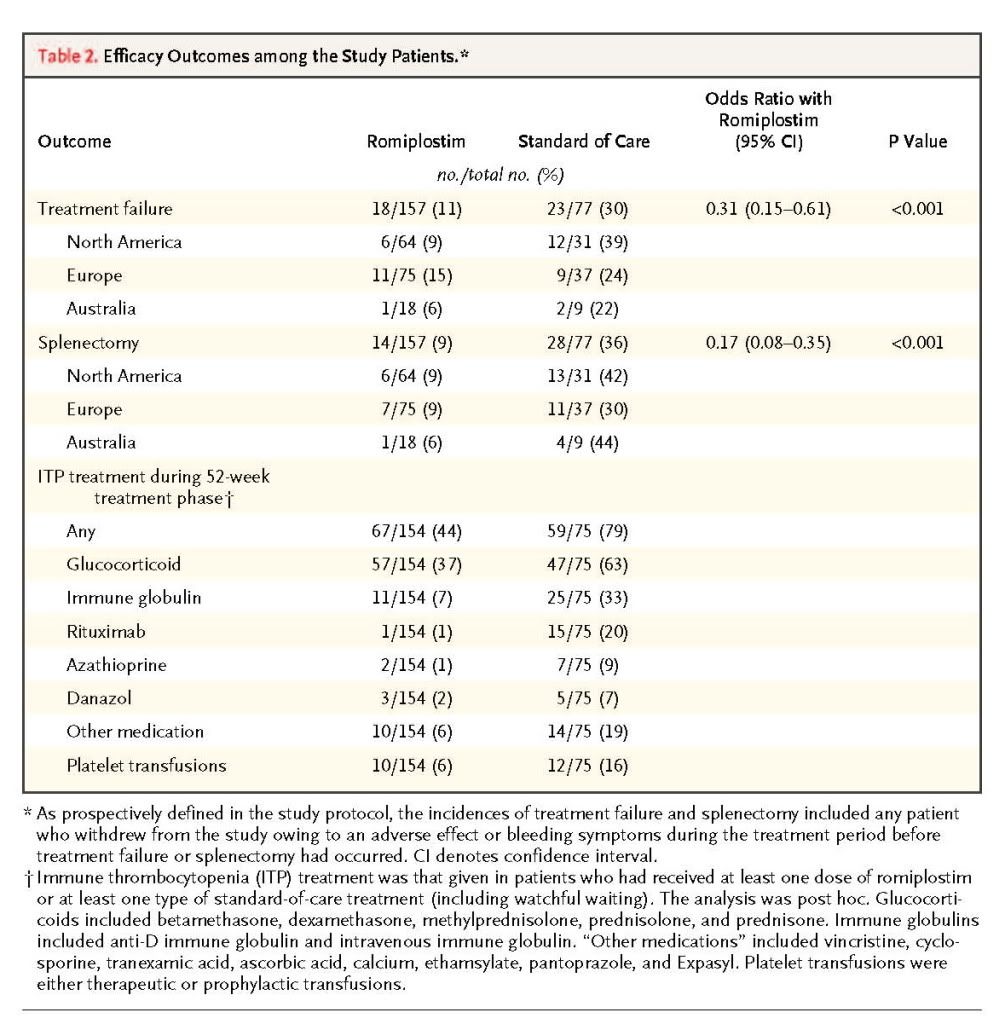
glucocorticoids were the most commonly administered. Overall, treatments were given in a much smaller proportion of patients in the romiplostim group (44%) than in the standard-of-care group (79%).
Between weeks 2 and 52, the percentage of patients with a platelet response (platelet count >50×109 per liter at any scheduled visit) ranged from 71% (108 of 152 patients) to 92% (127 of 138 patients) in the romiplostim group (median platelet count, 108×109 to 176×109 per liter) and from 26% (16 of 62 patients) to 51% (26 of 51 patients) in the standard-of-care group (median platelet count, 35×109 to 52×109 per liter). Patients in the romiplostim group were 2.3 times as likely to have a platelet response as those in the standard-of-care group (95% confidence interval, 2.0 to 2.6; P<0.001).
The mean romiplostim dose required to maintain the platelet count within the desired range (50×109 to 200×109 per liter) remained stable over time, particularly after the first 12 weeks of treatment
The mean (±SE) weekly dose was 3.9±2.1 μg per kilogram.
Treatment Failure and Splenectomy
The incidence of treatment failure was significantly lower among patients receiving romiplostim (18 of 157 [11%]) than among those receiving the standard of care (23 of 77 [30%], P<0.001) (Table 2). The reasons for treatment failure included major bleeding (in 3 patients receiving romiplostim and 6 receiving the standard of care), lack of efficacy (in 2 and 4 patients, respectively), and change of treatment owing to a severe side effect or bleeding (in 1 patient in each group); patients could have had more than one reason for treatment failure. The time to treatment failure was significantly longer in the romiplostim group than in the standard-of-care group (P=0.02)
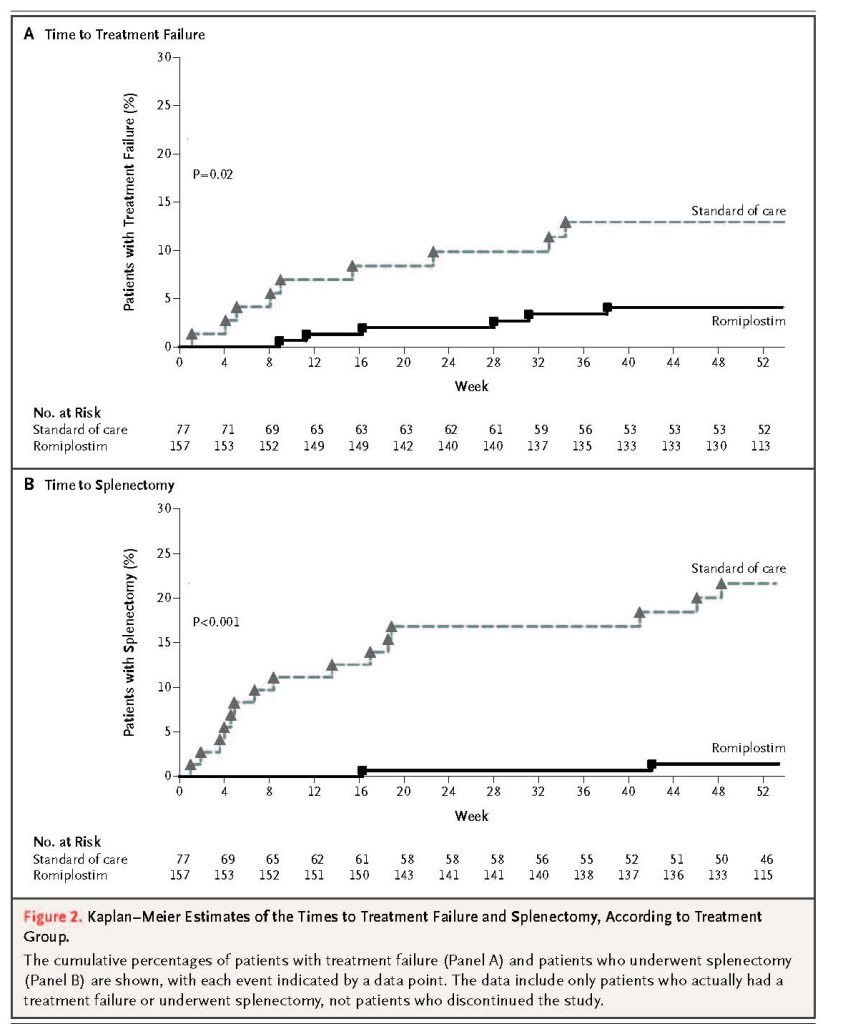
The incidence of splenectomy was significantly lower among patients receiving romiplostim (14 of 157 [9%]) than among those receiving the standard of care (28 of 77 [36%], P<0.001) (Table 2). The time to splenectomy was also significantly longer in the romiplostim group than in the standard-of-care group (P<0.001) (Figure 2B).
Geographic region had no significant effect on the incidence of treatment failure or splenectomy (Table 2). There was a significant, but weak, inverse correlation between the baseline platelet count (from a post hoc analysis) and the incidences of treatment failure and splenectomy (correlation coefficient for each end point, 0.16; P=0.01). The rate of treatment failure, across both groups, was less among patients whose immune thrombocytopenia was diagnosed 3 years or less before enrollment than among those whose immune thrombocytopenia was diagnosed more than 3 years before enrollment (P=0.03). No significant correlation was found between the incidence of splenectomy and any other baseline variable.
To assess the effect of study discontinuation on the primary end points, the patients who actually had incidences of treatment failure and splenectomy were also ascertained (Table 2 in theSupplementary Appendix). The results were similar to the estimated instances reported above.
Safety
Over 90% of patients in the two groups had at least one adverse event during the treatment period. Headache and fatigue were the most common. Serious adverse events occurred in 23% of patients (35 of 154) receiving romiplostim and in 37% of patients (28 of 75) receiving the standard of care, with treatment-related serious adverse events being reported in only 5% of patients (7 of 154) and 8% of patients (6 of 75), respectively. Serious adverse events for which the incidence was 1% or more in either group are listed in Table 3 in the Supplementary Appendix. Thrombocytopenia was most common, occurring in 3% of patients (5 of 154) receiving romiplostim and in 12% of patients (9 of 75) receiving the standard of care.
Adverse events of interest with thrombopoietin mimetics include bleeding, thrombosis, increased bone marrow reticulin, and hematologic cancer or myelodysplastic syndromes.
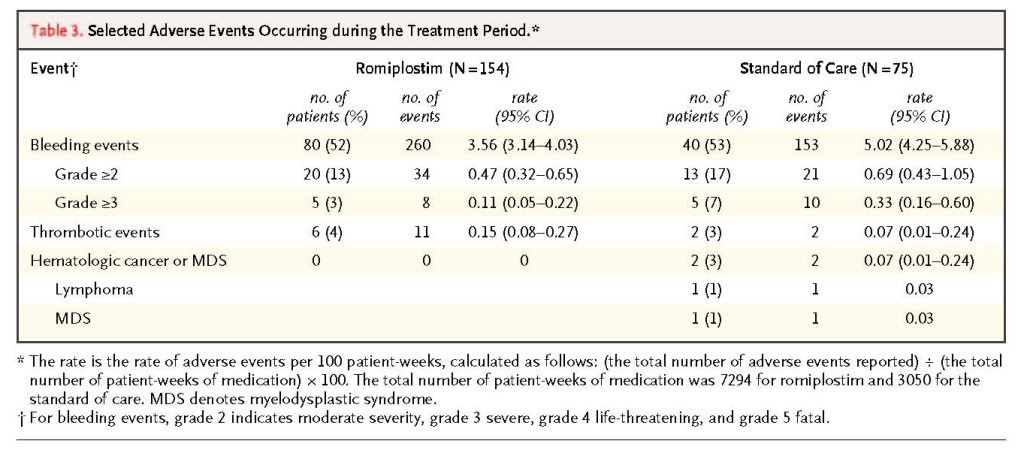
summarizes the incidence of these events (after adjustment for duration of exposure to the study drug). The romiplostim group had significantly lower adjusted incidences of overall bleeding events (P=0.001) and bleeding events of grade 3 or higher (P=0.02), as compared with the standard-of-care group. No significant differences between the two groups were noted with respect to less severe bleeding (P=0.17). Furthermore, 41 blood transfusions were administered to 12 of 154 patients (8%) receiving romiplostim, and 76 blood transfusions were administered to 13 of 75 patients (17%) receiving the standard of care. No significant between-group difference was found for thrombotic events. Two hematologic cancers occurred, both in the standard-of-care group. There were no abnormal nonhematologic laboratory results and no neutralizing antibodies against either romiplostim or thrombopoietin. Bone marrow reticulin was detected during the 6-month post-treatment safety monitoring period in 1 patient in the romiplostim group in whom reticulin had not been detected before treatment, but the level was within the normal range (grade 2).
Three deaths occurred during the treatment period: one in the romiplostim group (from pneumonia) and two in the standard-of-care group (one from hepatic failure and the other from cardiorespiratory arrest). Three additional deaths (from metastatic lung cancer, left ventricular failure, and hepatic neoplasm) occurred during the 6-month post-treatment safety monitoring period, all in patients receiving the standard of care. No deaths were considered to be related to treatment.
Quality of Life
No significant between-group differences were noted at baseline in the ITP-PAQ scores on any of the 10 scales. Scores on two scales — the Women's Reproductive Health and Work Quality of Life scales — could not be analyzed, owing to inadequacies in the statistical model. On the remaining eight scales (except the Fatigue scale), there were clinically significant increases in the scores at 52 weeks, as compared with baseline (i.e., increased by 8 to 15 points on a 100-point scale22), in both treatment groups (Table 4 in the Supplementary Appendix). The romiplostim group had significantly greater improvements in scores on these seven scales than did the standard-of-care group (P=0.01 for the Symptoms scale, P=0.008 for the Bother scale, P=0.02 for the Activity scale, P=0.049 for the Psychological scale, P<0.001 for the Fear scale, P=0.002 for the Social Quality of Life scale, and P=0.02 for the Overall Quality of Life scale), but the magnitude of the effect (between-group difference of 2 to 8 points) is of uncertain clinical benefit.22
DISCUSSION
Splenectomy is not considered an appropriate first-line therapy for most patients with immune thrombocytopenia,2 and many nonsurgical treatments either are poorly tolerated or fail to produce durable responses. In contrast, romiplostim increases and sustains platelet counts in patients with immune thrombocytopenia, with minimal toxic effects, for up to 5 years.18,19 Our results show that romiplostim not only maintains platelet counts more effectively than standard medical therapies but also reduces the overall rate of treatment failure and the need for splenectomy.
Since no single drug has been established as the standard treatment for immune thrombocytopenia, we compared romiplostim with a variety of standard-of-care therapies. The overall number of therapies used for immune thrombocytopenia was much higher in the standard-of-care group than in the romiplostim group (Table 2), which may be due to toxic effects or lack of efficacy.15,18,19
As might be expected from the rate of a platelet response with romiplostim, which was 2.3 times that with the standard of care, patients receiving romiplostim had fewer blood transfusions and a significantly lower rate of serious bleeding than patients receiving the standard of care. This resulted in a lower incidence of treatment failure with romiplostim (11%) than with the standard of care (30%). The most common reasons for treatment failure in both groups were bleeding and lack of efficacy.
The higher rate of a platelet response and lower incidence of treatment failure in the romiplostim group were also associated with a marked reduction in the need for splenectomy, which was performed in 9% of patients in the romiplostim group and 36% of patients in the standard-of-care group. The time to splenectomy was also markedly prolonged with the use of romiplostim. Although it has been suggested that other therapies, such as rituximab,23 dapsone,24 or anti-D immune globulin,25 may delay or reduce the need for splenectomy, these findings have not been confirmed in prospective randomized studies. Avoidance of splenectomy may allow for a spontaneous remission in a substantial number of patients2 and may benefit those who are not surgical candidates.
Adverse events associated with romiplostim treatment were similar to those in previous studies,15,19 were generally mild or moderate in severity, and did not result in treatment discontinuation. The lower incidence of serious adverse events in the romiplostim group (occurring in 23% of patients) as compared with the standard-of-care group (37% of patients) indicates that romiplostim is a generally safe treatment option for patients, relative to other therapies for immune thrombocytopenia. Bone marrow reticulin has been detected in a small number of patients treated with thrombopoietin mimetics26,27 and was observed in one patient in the romiplostim group during the 6-month post-treatment safety monitoring period. There were no significant between-group differences, after adjustment for duration of study-drug exposure, in the rates of reticulin fibrosis, thrombosis, or hematologic cancer during the treatment period.
The greater efficacy and safety of romiplostim, as compared with the standard of care, was accompanied by greater improvements in quality of life. The magnitude of the effect varied substantially across the 10 scales used; it is somewhat uncertain whether there was a clinically significant overall improvement associated with romiplostim use.
Unlike splenectomy, which may cure up to two thirds of patients with immune thrombocytopenia,2,8romiplostim is not curative and maintains an adequate platelet count in most patients only until discontinuation, when platelet counts usually fall. Its cost and the need for weekly injections may limit its long-term use. Despite the minimal safety concerns even after 5 years of exposure,18unanticipated effects of long-term administration might yet be revealed. In contrast to our previous studies, in which the median platelet count at enrollment was 19×109 per liter,15,19 in the current study, the median platelet count at enrollment was 29×109 per liter, suggesting that the patients had earlier, milder disease.
In conclusion, among patients with immune thrombocytopenia who received treatment for52 weeks, as compared with the standard of care, romiplostim was associated with higher rates of a platelet response, lower rates of treatment failure and splenectomy, less bleeding, and less need for other medical treatments for immune thrombocytopenia. Romiplostim therapy was not accompanied by clinically significant adverse effects and was associated with improved quality of life.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 