Hugo R. Rosen, M.D.
N Engl J Med 2011; 364:2429-2438June 23, 2011
This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies is then presented, followed by a review of formal guidelines, when they exist. The article ends with the author's clinical recommendations.
A 45-year-old man undergoing a routine examination for life insurance is noted to have an aspartate aminotransferase level of 80 U per milliliter (normal range, 9 to 40) and an alanine aminotransferase level of 110 U per milliliter (normal range, 7 to 52). He reports a remote history of intravenous drug use. Tests for hepatitis C antibody and hepatitis B surface antibody are positive, and tests for hepatitis A and human immunodeficiency virus (HIV) antibodies are negative. Genotyping of the hepatitis C virus (HCV) reveals genotype 1b; the viral load is 2,460,000 IU per milliliter. The complete blood count is normal; the platelet count is 220×109 per liter. An abdominal ultrasonogram is normal. How should this patient's case be managed?
Interferon-Based Antiviral Therapy
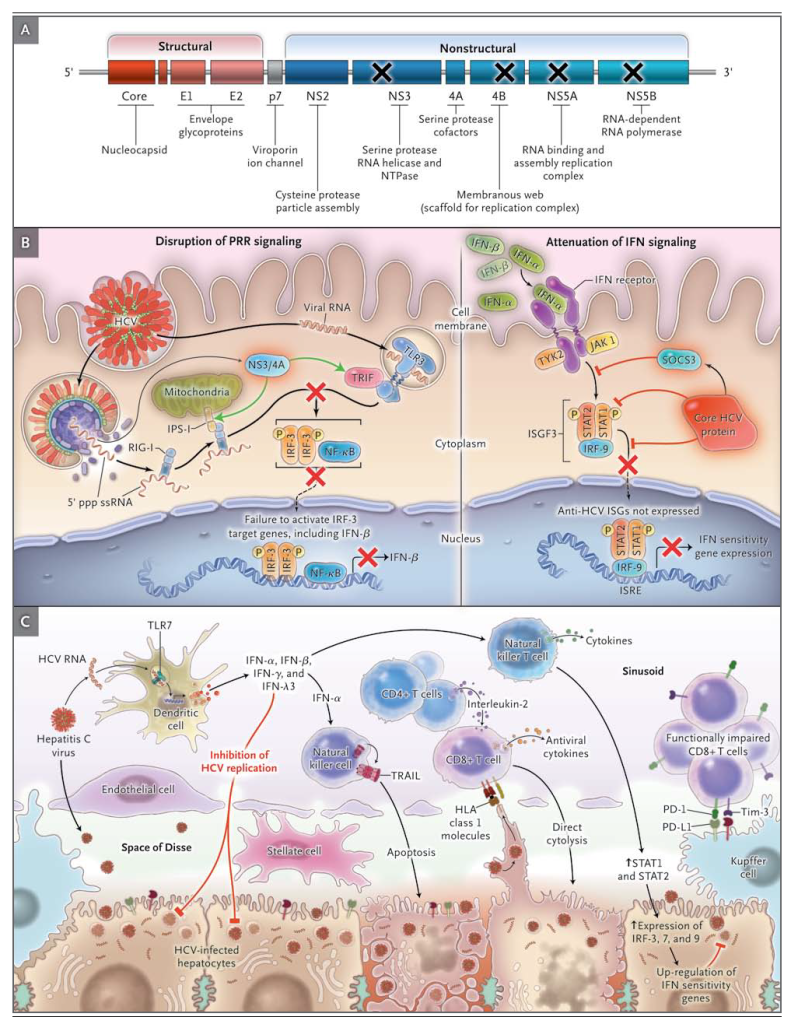
Substantial progress has been made in the treatment of HCV infection. The goals of therapy are to prevent complications and death from HCV infection; regardless of the stage of fibrosis, symptomatic extrahepatic HCV (e.g., cryoglobulinemia) is an indication for therapy.2 Over the past decade, on the basis of considerable data from randomized trials, pegylated interferon (peginterferon) plus ribavirin became the standard of care for all HCV genotypes.24-26
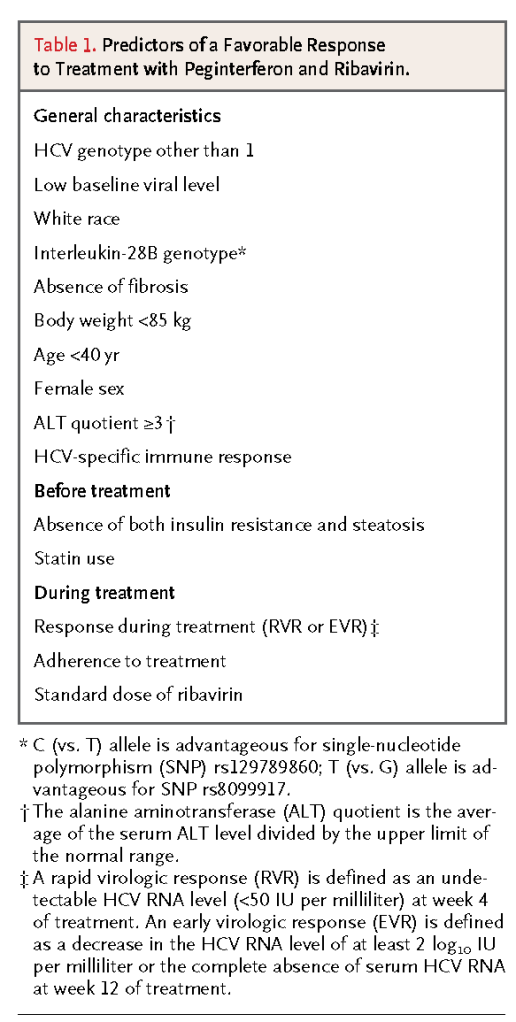
The two licensed peginterferons (Pegasys, Roche, and PegIntron, Merck) have been shown in head-to-head comparison to be equivalent in efficacy and to have similar safety profiles.27 Among patients with genotype 1 who are treated with peginterferon at the standard weight-based dose of ribavirin (1000 or 1200 mg per day) for 48 weeks, 40 to 50% have a sustained virologic response (defined as an undetectable HCV RNA level 24 weeks after the cessation of antiviral therapy). A shorter course of treatment and a lower ribavirin dose are associated with lower rates of sustained virologic response (and higher relapse rates) among genotype 1–infected patients.24-26 In contrast, patients with genotype 2 or 3, who account for approximately one quarter of HCV-infected patients in the United States, have rates of sustained virologic response in the range of 70 to 80% after taking peginterferon and ribavirin at a reduced dose (800 mg per day) for 24 weeks.25 A sustained virologic response is associated with permanent cure in the vast majority of patients.


GUIDELINES
The American Association for the Study of Liver Diseases2 and the American Gastroenterological Association49 have published guidelines for the assessment and management of chronic HCV infection, but these guidelines were issued before the publication of data from randomized trials of directly acting antiviral agents. Newer European guidelines take these data into account50; the recommendations provided below are generally consistent with these guidelines.
CONCLUSIONS AND RECOMMENDATIONS
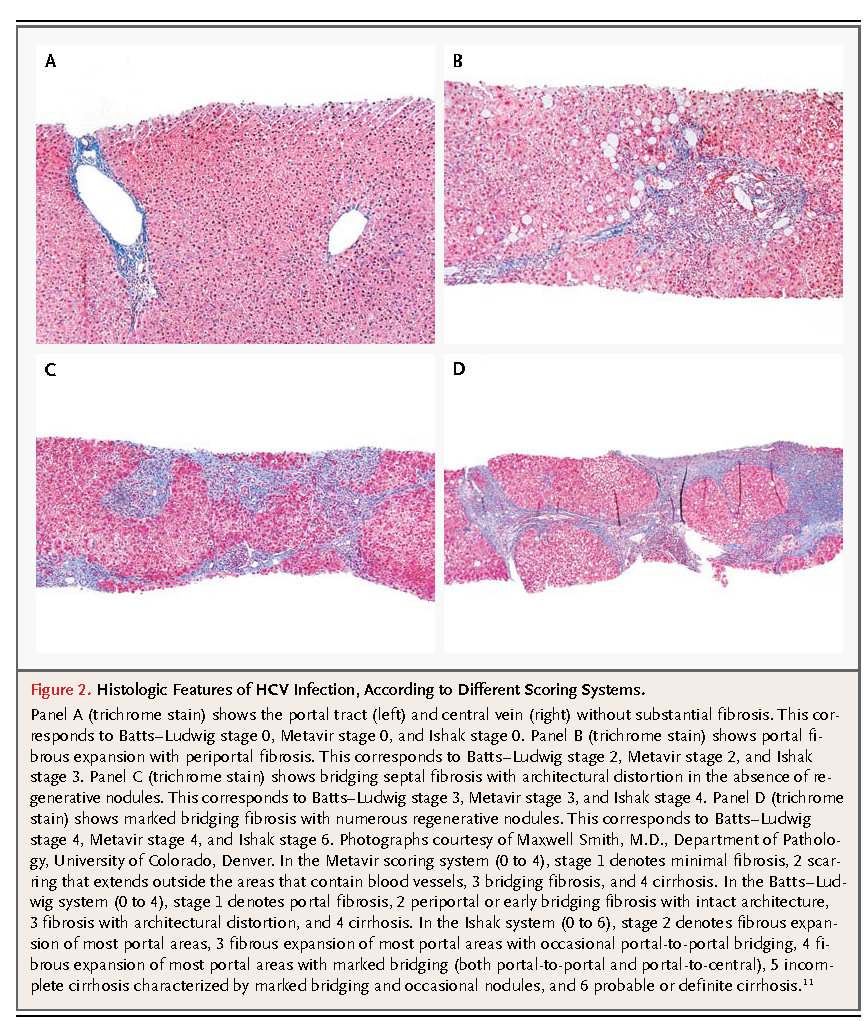
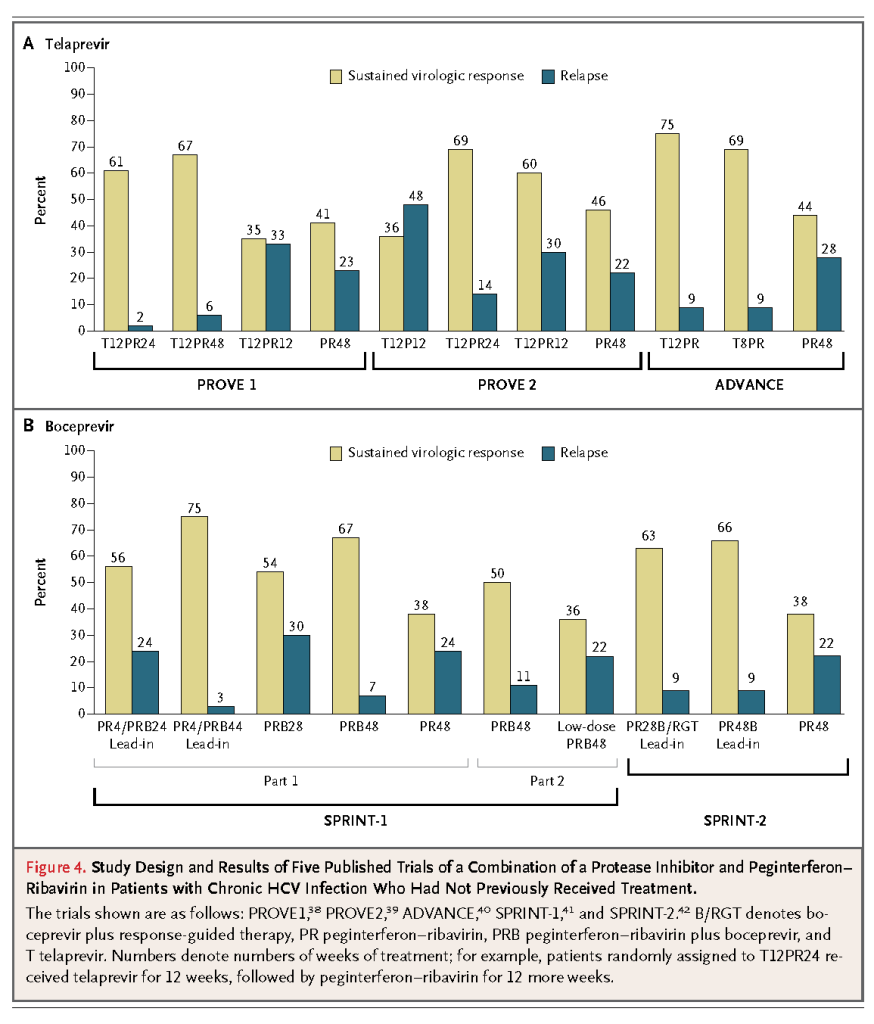
The patient described in the vignette has HCV genotype 1 with a high viral load. He should be vaccinated for hepatitis A because of an increased risk of liver failure among patients with chronic hepatitis C infection; hepatitis B vaccination is also indicated in those without evidence of prior exposure.2 Possible contraindications to treatment (e.g., depression) should be determined, and the patient should be informed about potential side effects of antiviral therapy.31,37-39,41,42 Although some clinicians would administer treatment without performing a liver biopsy, I would recommend a biopsy to assess the degree of fibrosis.31 For a patient with clinically significant fibrosis (Metavir score ≥2), triple antiviral therapy with peginterferon–ribavirin and an NS3/4A protease inhibitor, either telaprevir or boceprevir, should be recommended.
On the basis of data from recent randomized trials, a reasonable initial regimen would be telaprevir with peginterferon–ribavirin for 12 weeks. If tests for HCV RNA were negative at weeks 4 through 12 (indicating an extended rapid virologic response), only 12 additional weeks of peginterferon–ribavirin would be recommended, whereas if an extended rapid virologic response were not achieved, peginterferon–ribavirin would be continued for an additional 36 weeks.37 If boceprevir were used, according to new FDA guidelines, a 4-week lead-in phase of peginterferon–ribavirin would be followed by peginterferon–ribavirin and boceprevir for 24 weeks (a total of 28 weeks) if tests for HCV RNA were negative at weeks 8 through 24 of treatment. If the tests were positive between weeks 8 and 24 but negative at week 24, peginterferon–ribavirin and boceprevir would be continued for an additional 8 weeks, followed by an additional 12 weeks of peginterferon–ribavirin (a total of 48 weeks).
Alternatively, if the patient has milder fibrosis and is reluctant to receive treatment, it would be reasonable to wait and reevaluate as new therapeutic agents become available.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 