Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. May 1, 2014.
[Free full-text DHHS Guideline PDF]
Key Updates to Existing Sections
The following are key updates to existing sections of the guidelines.
Change in Recommendations on Frequency of CD4 Count Monitoring
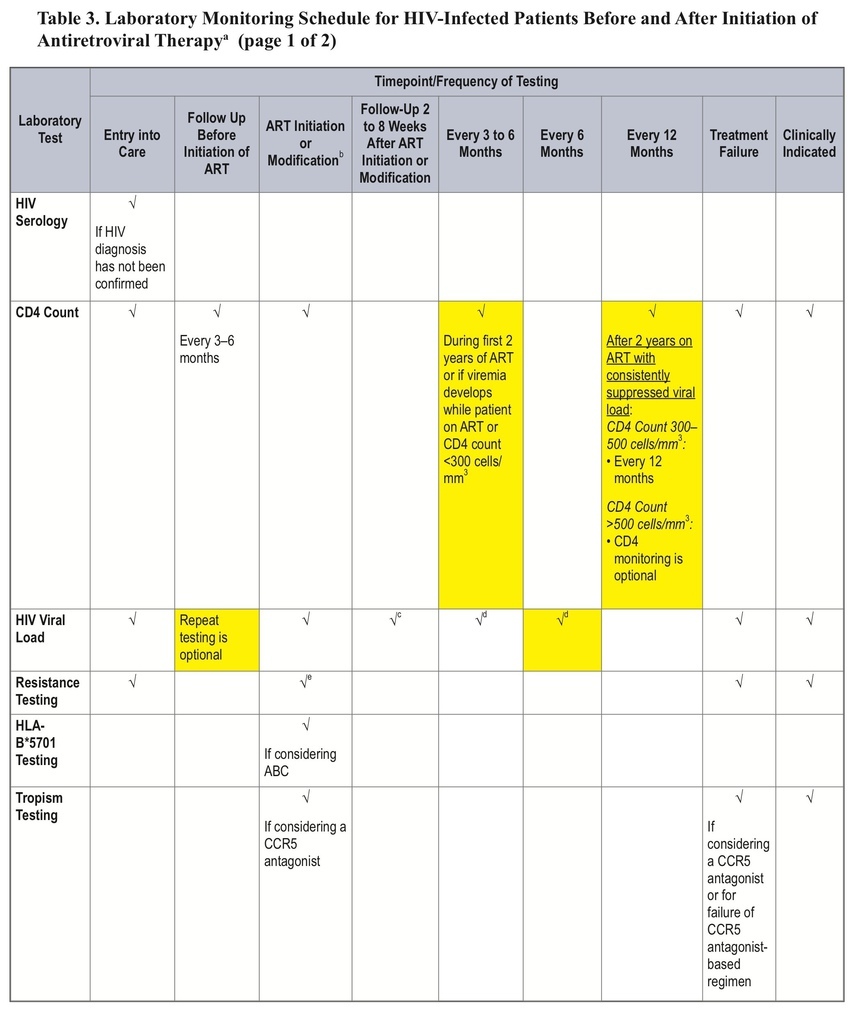
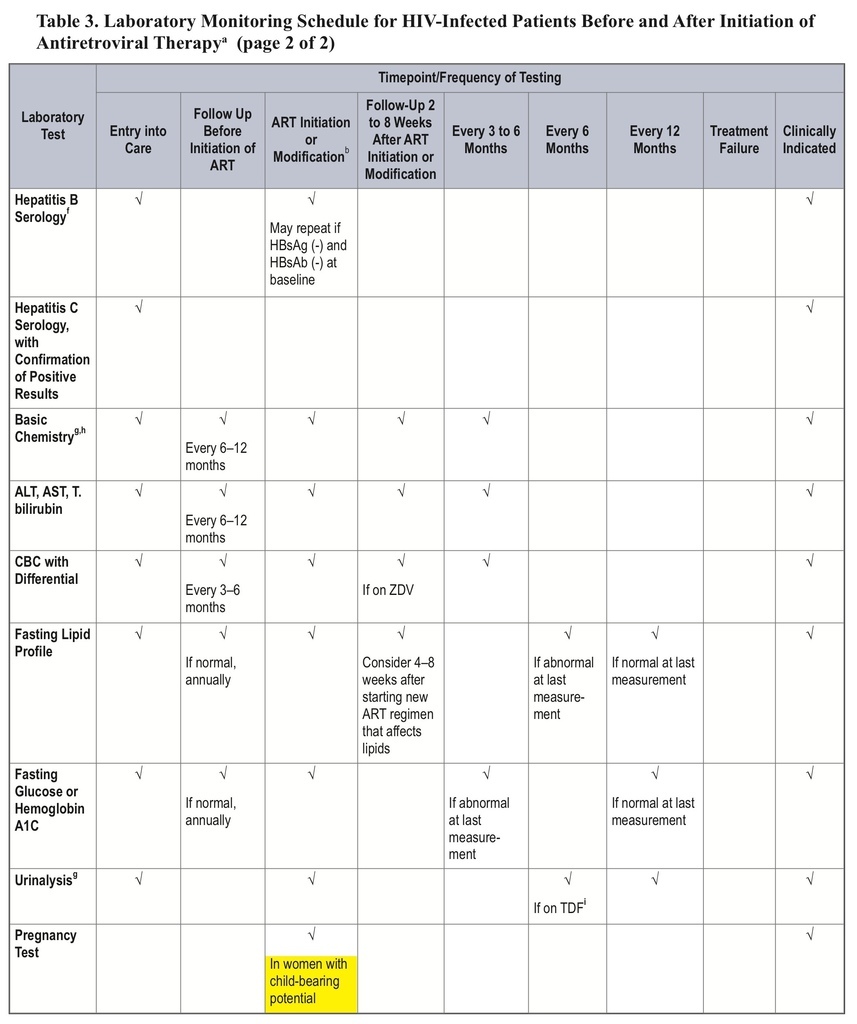
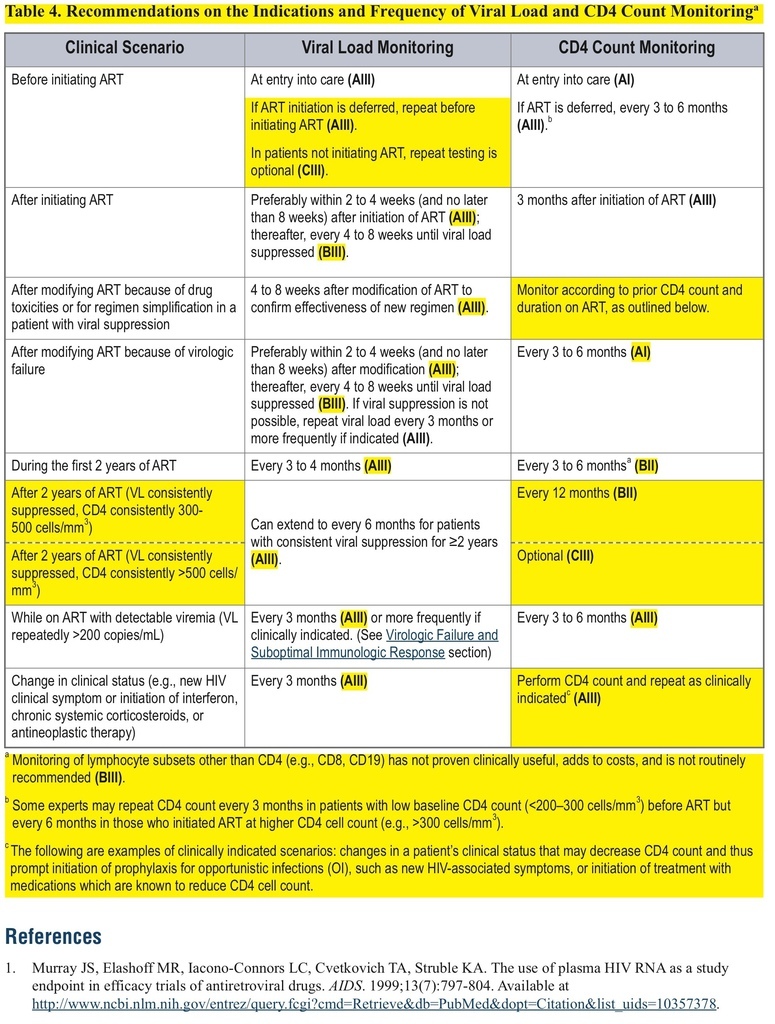
This change can be found in the “Laboratory Testing: HIV-1 RNA (Viral Load) and CD4 Count Monitoring” section.
This major revision includes a recommendation for less-frequent CD4-count monitoring, an updated list of recommended regimens, a discussion of “switching,” and a new section on treatment costs.
Sponsoring Organization: U.S. Department of Health and Human Services
Target Population: Healthcare providers involved in the treatment of HIV infection
Background and Objective
This document updates guidelines last revised in February 2013.
What's Changed
• The recommended frequency of CD4-count monitoring has been decreased for patients on antiretroviral therapy (ART) for >2 years with consistent viral suppression.
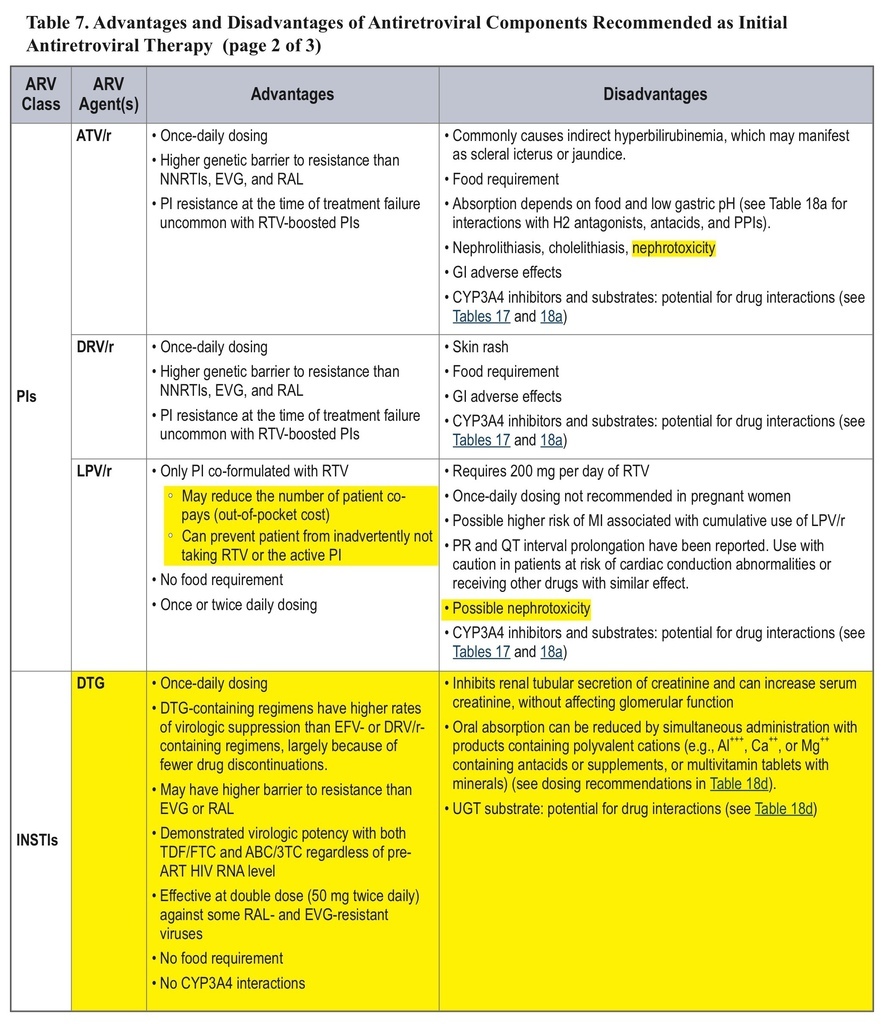
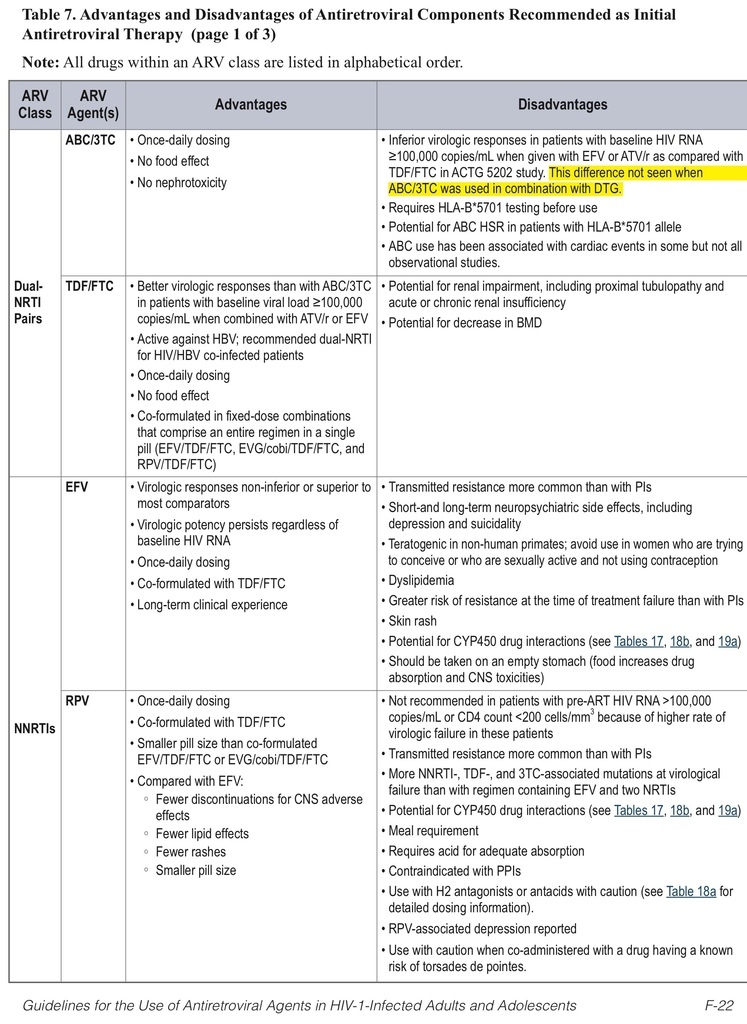
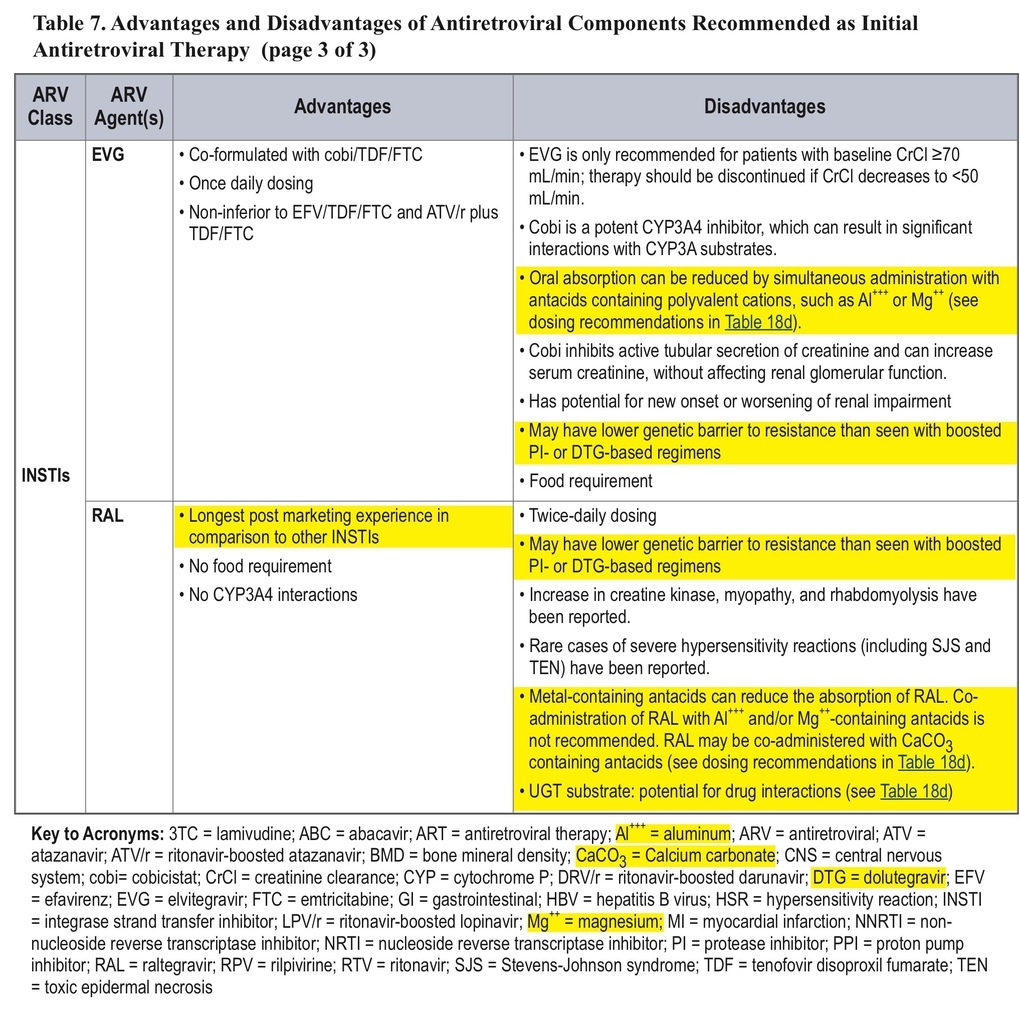
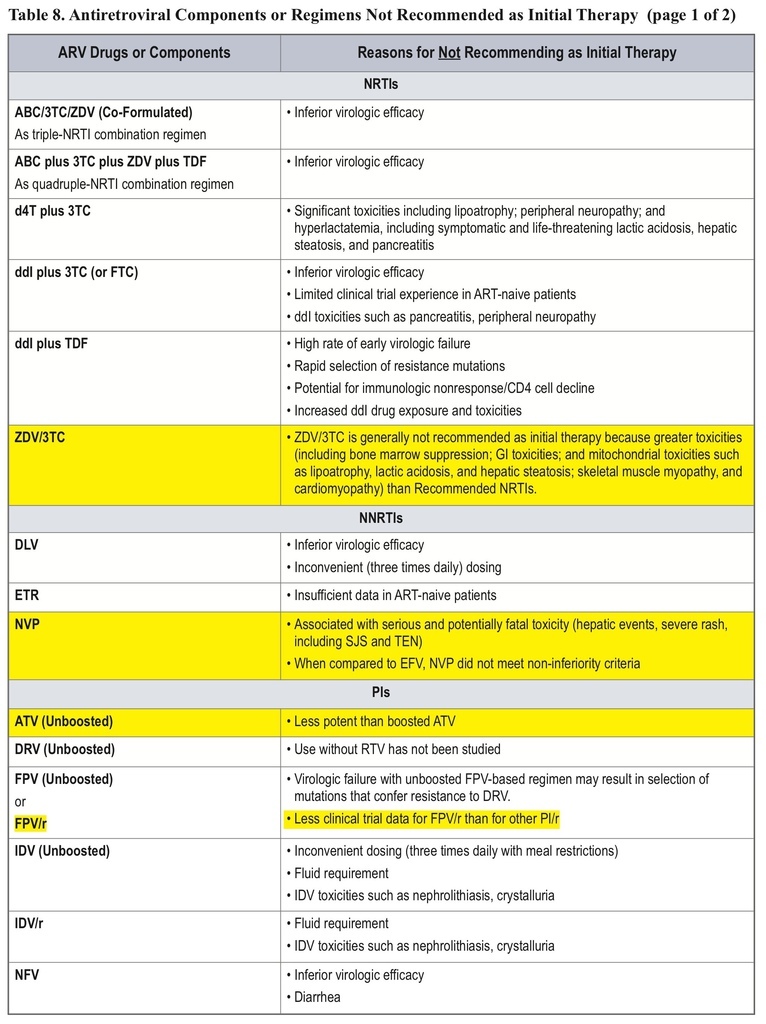
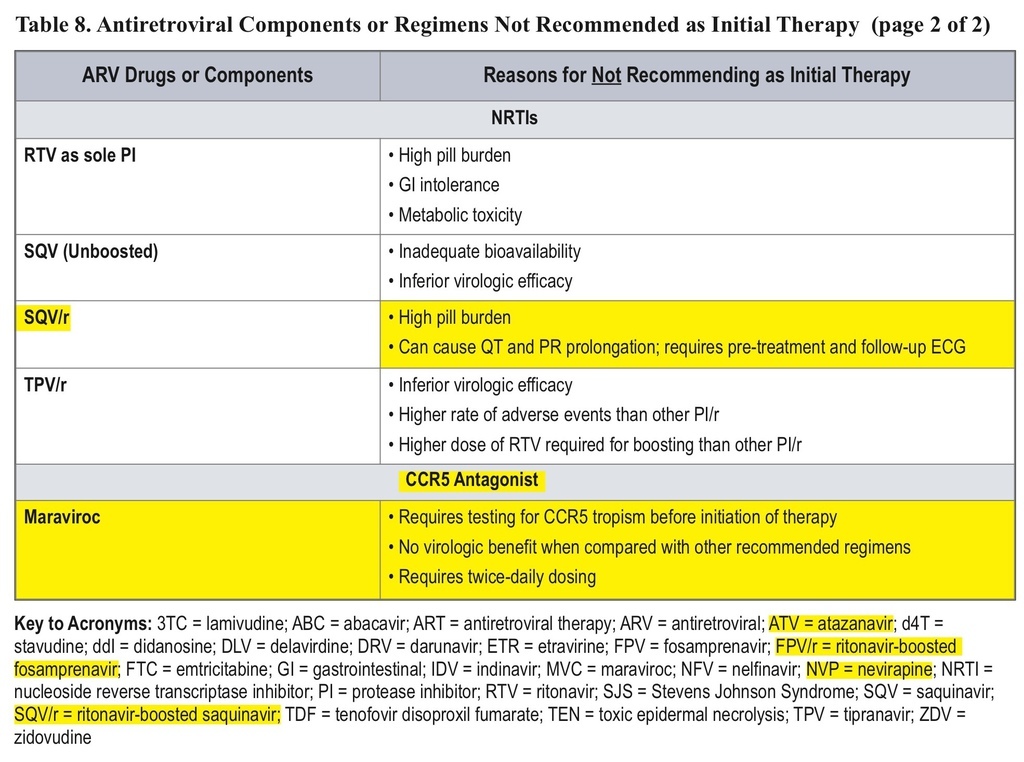
• The classification of initial combination ART regimens has been changed from “preferred” to “recommended,” and the number of recommended regimens has been increased. Some older drugs are no longer recommended for initial therapy.
• “Regimen Simplification” has been updated to “Regimen Switching in the Setting of Viral Suppression,” with guidance on key principles underlying this option.
• A new section on cost considerations in ART has been added.
Key Points
• For patients on ART for >2 years with consistent viral suppression, CD4-count monitoring should occur every 12 months (at counts 300–500 cells/mm3) or be considered optional (at counts >500 cells/mm3).
• Over and above the previously listed seven “preferred” initial ART regimens, three new options have been added for patients with pretreatment HIV RNA <100,000 copies/mL, with caveats about checking HLA-B*5701 (regimens with abacavir) or use only in patients with CD4 counts >200 cells/mm3(regimens with rilpivirine). This larger list, relabeled as “recommended” regimens, now includes efavirenz + abacavir/3TC, rilpivirine/tenofovir/FTC, and ritonavir-boosted atazanavir + abacavir/3TC.
• AZT, nevirapine, unboosted atazanavir, ritonavir-boosted fosamprenavir or saquinavir, and maraviroc are no longer recommended for initial therapy.
• The key principle in switching ART regimens in the setting of viral suppression is maintaining suppression without compromising future options. Consideration of a switch should include review of the patient's treatment history, responses to ART, resistance profiles, and drug tolerance.
• To optimize clinical outcomes in the current healthcare environment, providers should consider cost as one factor among many in selecting a particular ART regimen. The guidelines include discussions of costs relative to adherence, the role of generic antiretrovirals, requirements for prior authorization for some drugs, the costs of lab testing, and strategies for containing costs without compromising treatment efficacy, as well as a table showing average wholesale prices for antiretroviral drugs.
COMMENT
This major revision to the Department of Health and Human Services' HIV treatment guidelines is timely and comprehensive. The changes described above and the new recommendations and sections capture current practices and standards. This document is an outstanding reference for all healthcare providers who treat HIV-infected individuals.
Charles B. Hicks, MD





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 