Focal segmental glomerulosclerosis accounts for approximately 20% of cases of the nephrotic syndrome in children and 40% of such cases in adults, with an estimated incidence of 7 per 1 million.
It is the most common primary glomerular disorder causing end-stage renal disease in the United States, with a prevalence of 4%.2 The cardinal feature is progressive glomerular scarring. Early in the disease course, glomerulosclerosis is both focal, involving a minority of glomeruli, and segmental, affecting a portion of the glomerular globe. With progression, more widespread and global glomerulosclerosis develops. Since the first clinical–pathological studies of the disease in the 1970s,3 there has been renewed interest because of the increasing incidence of the disease,4better understanding of causation, and identification of the podocyte as the major cellular target.5The discovery that mutations in podocyte genes are associated with genetic focal segmental glomerulosclerosis has advanced the field of podocyte biology and stimulated new approaches to diagnosis and management.
CLINICAL FEATURES
Proteinuria is a defining feature of focal segmental glomerulosclerosis, typically accompanied by hypoalbuminemia, hypercholesterolemia, and peripheral edema. The nephrotic syndrome in children is defined as proteinuria (>1 g of urine protein per square meter of body-surface area per day), hypoalbuminemia (<2.5 g of albumin per deciliter), hypercholesterolemia (>200 mg of total cholesterol per deciliter), and edema. In adults, the nephrotic syndrome is defined as a urine protein level of more than 3.5 g per day and an albumin level of less than 3.5 g per deciliter. Approximately 75 to 90% of children and 50 to 60% of adults with focal segmental glomerulosclerosis have the nephrotic syndrome at presentation.
Once considered a single disease, focal segmental glomerulosclerosis is now viewed as a group of clinical–pathologic syndromes sharing a common glomerular lesion and mediated by diverse insults directed to or inherent within the podocyte
Despite the identification of many factors that lead to focal segmental glomerulosclerosis, approximately 80% of cases are primary (idiopathic). Focal segmental glomerulosclerosis and a related disorder, minimal change disease, are quintessential podocyte diseases, or “podocytopathies.”7,8 In both conditions, podocyte injury leads to effacement of the podocyte foot processes, which is the major structural correlate of nephrotic proteinuria. This change in podocyte shape requires rearrangement of the actin cytoskeleton, a process that is typically reversible with glucocorticoid therapy in minimal change disease but irreversible and progressive in focal segmental glomerulosclerosis.
PATHOGENESIS
Loss of Filtration Barrier
Nephrotic proteinuria results from loss of integrity of the glomerular filtration barrier, which regulates permselectivity through the intimate association of three layers: fenestrated glomerular endothelial cells at the inner blood interface, the glomerular basement membrane in the center, and podocytes (also known as visceral epithelial cells) at the outer urinary interface
Podocytes are highly differentiated, polarized epithelial cells resembling neurons in their large cell body and elongated cellular extensions, stabilized by a central actin cytoskeleton core
The foot processes interdigitate along the outer aspect of the glomerular capillary wall, linked to their neighbors by slit diaphragms, which are modified adherens junctions aligned in a zipperlike array.9 Podocytes provide structural support to the glomerular capillaries and synthesize the proteins of the slit diaphragm and many extracellular matrix components of the glomerular basement membrane. These terminally differentiated cells cannot repair by means of cell division, making podocyte depletion through detachment, apoptosis, or necrosis a critical mediator of glomerulosclerosis.8 In the past decade, new insights have derived both from animal models of podocyte depletion and genetic studies of human disease.
Podocyte Depletion in Experimental Toxin Models
Experimental models have addressed whether delivery of a lethal toxin specifically and exclusively to the podocyte is sufficient to cause focal segmental glomerulosclerosis. For example, the creation of a transgenic animal that expresses a toxin receptor under the control of a podocyte-specific promoter permits the targeting of a toxin exclusively to podocytes.10,11 In such a model, internalization of diphtheria toxin or pseudomonas exotoxin A kills podocytes by the inhibition of protein synthesis. The degree of podocyte depletion after toxin exposure correlates closely with the severity of disease in these models.11 Loss of more than 40% of podocytes leads to overt focal segmental glomerulosclerosis with high-grade proteinuria and renal insufficiency, indicating a disease threshold.11 Podocytes are shed into the urine for months after a brief toxin exposure, suggesting a secondary autonomous phase of podocyte loss.12 In a chimeric model in which only a subset of podocytes express toxin receptor, podocyte injury and dedifferentiation are observed to spread to neighboring toxin-resistant podocytes that escaped the initial insult.13 This chimeric model suggests that injury can propagate locally from podocyte to podocyte by a domino-like effect, which may explain the segmental nature of the lesions. Although the mediators are unknown, a secondary wave of podocyte injury hypothetically might decrease podocyte survival factors that signal through nephrin and glutamate receptors14 or might increase noxious factors, such as shear stress, angiotensin II, or transforming growth factor β (TGFβ).13
An experimental model of focal segmental glomerulosclerosis that is induced by the anthracycline doxorubicin (also called adriamycin) causes severe disease in BALB/c mice, whereas other mouse strains are protected. The strain dependence went unexplained until the recent discovery of the susceptibility gene as an ancestral mutation in Prkdc (protein kinase, DNA-activated, catalytic polypeptide), which encodes a component of the DNA double-strand break-repair machinery.15 In BALB/c mice, there is no nonhomologous end-joining DNA repair after intercalation of doxorubicin into podocyte DNA, leading to mitochondrial DNA depletion.15 This murine model illustrates the importance of protective mechanisms against genotoxic stress to enhance podocyte longevity.
Genetic Susceptibility
Since the discovery of nephrin as the major component of the slit diaphragm in 1998,16 the number of identified podocyte mutations in familial and sporadic focal segmental glomerulosclerosis has grown (Figure 2, and the table in the Supplementary Appendix, available with the full text of this article at NEJM.org). The genes encode diverse podocyte products located in the slit diaphragm,16-19 cell membrane,20-24 cytosol,25 actin cytoskeleton,26-29 nucleus,30,31 mitochondria,32-34 and lysosomes.35 Mutations in nephrin and podocin are the most frequent.36 Most mutations follow an autosomal recessive transmission and manifest early in life. Autosomal dominant forms (e.g., mutations in genes encoding α-actinin-4 and transient receptor potential cation channel 6) usually present in late adolescence or adulthood. Many of the genes that are involved were identified by positional cloning in affected families and later validated in global or podocyte-specific knockout models or in transgenic models that express the mutated genes. Genetic defects have been identified in up to two thirds of patients with focal segmental glomerulosclerosis who present in the first year of life, underscoring the importance of genetic testing in this age group.37 Genetic testing is most likely to uncover a basis for focal segmental glomerulosclerosis in infants, young children, and patients with syndromic disease or a positive family history. A small but clinically important percentage of older children and adults with sporadic glucocorticoid-resistant disease may also harbor mutations.36
Podocyte genes encode diverse structural proteins or enzymes that participate in signaling events that regulate podocyte growth, differentiation, motility, and interactions between cells and between cells and matrix.38 These gene products are coupled to the actin cytoskeleton directly or indirectly through intermediary proteins (Figure 2). Disruption or dysregulation of signaling through these proteins leads to rearrangement of the actin cytoskeleton and the generalized response of foot-process effacement. The podocyte is a motile cell endowed with mechanosensors that respond to positional stimuli and shear stress.39 Factors that promote the development of a cytoskeleton that is either too rigid or too dynamic pose potential threats to podocyte survival. For example, disease-causing mutations in α-actinin-4 produce a rigid cytoskeleton by exposing a buried actin-binding site that is independent of calcium regulation, leading to a gain of function.40 Once the actin cytoskeleton undergoes rearrangement, the loss of foot-process anchoring may weaken podocyte attachments to the glomerular basement membrane, rendering them more vulnerable to detachment in response to filtration pressures. It is likely that the wear and tear from shear stress, stretch tension, oxidative stress, and DNA damage that accrues over years may compound a genetic basis for this disease.41 Such accumulated second hits might explain the late onset of genetic focal segmental glomerulosclerosis in adults with autosomal dominant mutations.
Genetic Basis in Patients of African Descent
Across age groups, the incidence of focal segmental glomerulosclerosis is higher and the rate of renal survival is worse among blacks than among whites. Mapping by means of admixture linkage disequilibrium in large populations identified genetic risk factors for focal segmental glomerulosclerosis and end-stage renal disease in blacks. Two genes in close linkage disequilibrium were identified on human chromosome 22. MYH9, encoding myosin heavy chain 9, a nonmuscle myosin IIA that is a component of the podocyte cytoskeleton, was identified first42 and was an attractive candidate because MYH9 mutations were known to cause a rare autosomal dominant form of focal segmental glomerulosclerosis in patients with Epstein–Fechtner syndromes (renal disease, sensorineural deafness, and macrothrombocytopenia).27,43 Genomewide scans identified three single-nucleotide polymorphisms in intron 23 of the MYH9 gene as conferring risk for primary focal segmental glomerulosclerosis and hypertensive end-stage renal disease among blacks in an autosomal recessive model.42,44 However, further probing of the genetic interval found two independent sequence variants (called G1 and G2) in the last exon of the neighboring gene encoding apolipoprotein L1 (APOL1) that had a stronger association with focal segmental glomerulosclerosis, with a combined signal that was increased by a factor of 35 over that of MYH9. Thus, APOL1 was implicated as the actual susceptibility gene.45
Selection tests in Europeans and Africans showed that the APOL1 G1 and G2 haplotypes were under strong selection only in Africa.45 Apolipoprotein L1 is a plasma factor that can lyseTrypanosoma brucei brucei, the parasite that causes sleeping sickness. Two subspecies of trypanosoma that are resistant to lysis by apolipoprotein L1, T. brucei rhodesiense and T. brucei gambiense, evolved in sub-Saharan Africa. The G1 and G2 variants of APOL1 lyse T. brucei rhodesiense, but not T. brucei gambiense, a finding that explains how these variants could have risen to high frequency by natural selection. This scenario is analogous to sickle cell trait, in which a mutation in the hemoglobin A beta chain confers protection against malaria but at the risk of hemoglobinopathy. In both situations, the protection against parasitic infection is a dominant trait present in heterozygotes, whereas the development of host disease is a recessive trait, present in homozygotes. How the APOL1 G1 and G2 variants act mechanistically on the podocyte to cause focal segmental glomerulosclerosis has not been delineated.
PATHOLOGICAL DEFINITION
The histologic definition of focal segmental glomerulosclerosis is a segmental obliteration of glomerular capillaries by extracellular matrix.7,46 Entrapment of plasma proteins as hyalinosis commonly accompanies the sclerosis. Because juxtamedullary nephrons are often affected first, adequate glomerular sampling is needed to identify the diagnostic lesions. Adhesions or synechiae may form between the sclerosing segment and Bowman's capsule. On electron microscopy, the major finding is extensive effacement of the foot processes without other abnormalities in the glomerular basement membrane. Detachment of podocytes from the glomerular basement membrane occurs in regions overlying the sclerotic lesions. At these sites, there is often accumulation of loose matrix material synthesized by parietal cells that migrate onto the tuft, producing a halolike effect. Granular immune-type electron-dense deposits are not present. Immunofluorescence typically reveals coarse segmental staining for IgM and C3 entrapped in areas of hyalinosis. As individual nephrons degenerate, tubular atrophy and interstitial fibrosis develop. Proximal tubular reabsorption droplets reflect heightened tubular trafficking of albumin and lipoproteins, a process that contributes to progressive tubulointerstitial injury.47
The pathologic diversity of glomerular lesions in focal segmental glomerulosclerosis is evident.7,46,48 Lesions differ anatomically in their location with respect to the glomerular hilus (vascular pole) and the tubular pole and qualitatively with respect to glomerular hypercellularity and capillary collapse.46 A classification of histologic variants recognizes not-otherwise-specified (NOS),46 perihilar,46,49-53 cellular,53,54 tip,46,55,56 and collapsing disease57-62 variants and is applicable to both primary and secondary focal segmental glomerulosclerosis
In the collapsing variant, podocytes have an immature, dysregulated phenotype.63,64 Because injured podocytes lose differentiation markers such as nephrin, the identity of the cells that proliferate in Bowman's space has been controversial. Recent studies using parietal-cell markers suggest that most of these cells actually originate from the parietal layer.65-67Moreover, progenitor cells bearing stem-cell markers CD133 and CD24 line Bowman's capsule, possibly serving as a reservoir to replenish lost podocytes.68 Although progenitor cells may be recruited to sites of podocyte denudation, it is not known whether they can differentiate into the mature podocytes that are needed to reconstitute a normal filtration barrier.
PRIMARY (IDIOPATHIC) DISEASE
Primary focal segmental glomerulosclerosis has long been attributed to a putative circulating permeability factor. Indirect evidence for a circulating plasma factor includes the ability to modulate proteinuria by immunoadsorption, potential disease recurrence minutes after renal transplantation, and therapeutic reduction in proteinuria by plasmapheresis.69 In addition, serum samples from patients with focal segmental glomerulosclerosis cause increased permeability to albumin in isolated glomeruli and induce foot-process effacement and proteinuria when injected into rats. Several candidate plasma factors have been proposed. For example, cardiotrophin-like cytokine 1, a member of the interleukin-6 family, has permeability activity in a plasma fraction with a molecular weight of less than 30 kD and can be enriched by means of galactose affinity chromatography.69Elevated serum levels of soluble urokinase receptor (>3000 pg per milliliter) have been identified in up to two thirds of patients with primary focal segmental glomerulosclerosis but not in those with minimal change disease.70 Increased serum levels of soluble urokinase receptor before renal transplantation were associated with an increased risk of recurrent disease in the allograft.70Circulating soluble urokinase receptor induces foot-process effacement through the activation of podocyte β3 integrin, and its effect can be blocked in animal models by neutralizing antibodies targeting soluble urokinase receptor.70,71 The cellular source and stimulants of soluble urokinase receptor in patients with focal segmental glomerulosclerosis are unknown.
VIRUS-INDUCED DISEASE
Viruses can act on the podocyte either by direct infection or by the release of inflammatory cytokines that interact with podocyte receptors. The best studied of such viruses is human immunodeficiency virus type 1 (HIV-1), which directly infects podocytes and tubular epithelial cells.72 Evidence supports HIV-1 entry by transfer from infected T cells to tubular epithelial cells through virologic synapses formed during cell adhesion, independent of CD4.73 HIV-1 can persist in the kidney epithelium despite antiretroviral therapy and normalization of peripheral CD4 counts. HIV-1 gene expression by infected renal epithelium in turn induces dysregulation of host genes. The form of focal segmental glomerulosclerosis associated with untreated HIV-1, called HIV-associated nephropathy (HIVAN), typically progresses rapidly and is associated with glomerular collapse.62 In vivo and in vitro models have identified viral genes nef and vpr as particularly important in HIVAN pathogenesis.74,75 Nef, a virulence factor, contains a proline-rich motif that interacts with the SH3 domain of the Src family kinases. Through downstream activation of STAT3 and MAPK1/2, it promotes podocyte dedifferentiation and proliferation, whereas interaction with diaphanous interacting protein mediates the up-regulation of Rac1, reduction in RhoA, and dysregulation of actin cytoskeleton.76 Vpr, which is required for nuclear entry of the HIV-1 preintegration complex, mediates tubular epithelial G2 cell-cycle arrest and apoptosis.77,78 Parvovirus B19 is another virus that can infect podocytes and tubular cells, leading to collapsing focal segmental glomerulosclerosis.60 Other viruses associated with this disease, such as simian virus 40, cytomegalovirus, and Epstein–Barr virus, are less well characterized.57,61
DRUG-INDUCED DISEASE
Historically, the first drug associated with focal segmental glomerulosclerosis was heroin, though the incidence of this drug-induced disease (known as heroin nephropathy) has fallen sharply in parallel with the increasing purity of modern street heroin.79 The bisphosphonate pamidronate, an osteoclast inhibitor used to reduce bone resorption in patients with myeloma and metastatic cancers, has been linked to the development of focal segmental glomerulosclerosis.58 Proteinuria and renal failure associated with pamidronate typically improve after withdrawal of the drug. Pamidronate has direct toxic effects on osteoclasts, including disruption of the actin cytoskeleton, suggesting the possibility of a similar effect on the podocyte cytoskeleton.
All forms of interferon therapy, including interferon alfa (widely used to treat hepatitis C), interferon beta (indicated for multiple sclerosis), and interferon gamma (formerly used in idiopathic pulmonary fibrosis and indicated for chronic granulomatous disease and malignant osteopetrosis), have been reported to induce focal segmental glomerulosclerosis. 59 The podocyte has receptors for interferon alfa and interferon beta and expresses major histocompatibility complex class II antigen in response to interferon gamma, suggesting potential direct podocyte effects. In the transplanted kidney, toxic effects from calcineurin inhibitors are associated with collapsing focal segmental glomerulosclerosis and hyaline arteriolopathy, probably through acute ischemia from severe vasoconstriction.80,81 In addition, the mammalian target of rapamycin (mTOR) inhibitor sirolimus (also known as rapamycin) can induce focal segmental glomerulosclerosis by reducing podocyte expression of critical proteins in the slit diaphragm and cytoskeleton, including nephrin.82
DISEASE SECONDARY TO HEMODYNAMIC ADAPTATIONS
Another form of focal segmental glomerulosclerosis, termed adaptive focal segmental glomerulosclerosis, is thought to result from structural and functional adaptations mediated by intrarenal vasodilatation, increased glomerular capillary pressures, and plasma flow rates.52 Such maladaptive responses may arise through a reduction in the number of functioning nephrons (e.g., in unilateral renal agenesis, reflux nephropathy, or low nephron endowment owing to very low birth weight50) or through mechanisms that place hemodynamic stress on an initially normal nephron population (e.g., in morbid obesity, cyanotic congenital heart disease, and sickle cell anemia) (Table 1). Unlike primary focal segmental glomerulosclerosis, adaptive disease is often associated with normal serum albumin levels, despite nephrotic-range proteinuria, and biopsy samples obtained from such patients often show enlarged glomeruli, perihilar sclerosis, and relatively mild degrees of foot-process effacement. 7,46
Animal models in which renal mass is markedly reduced have elucidated the mechanistic bases for adaptive focal segmental glomerulosclerosis.83 Reflex vasodilatation of both the afferent and efferent arterioles follows a marked reduction in renal mass, causing elevation in the flow rate in the glomerular capillaries. Because the reduction in vascular resistance is greater in the afferent arteriole than in the efferent arteriole, glomerular hydrostatic pressure rises, producing glomerular hypertension. These responses cause an elevation in the single-nephron glomerular filtration rate in proportion to the amount of kidney excised.52,83 Glomerular volume and surface area increase, placing mechanical strain on podocytes that stretch to cover the expanding tuft. Some hypertrophied podocytes detach, producing denuded patches of glomerular basement membrane. These sites become covered by parietal cells, leading to the formation of a synechia to Bowman's capsule and a nidus for the development of segmental sclerosis.84
Although this scenario is the initiating step in the adaptive forms of focal segmental glomerulosclerosis, it may supervene in the later stages of other forms of the disease. The loss of a critical number of nephrons promotes the activation of the renin–angiotensin system (RAS), exacerbating proteinuria and setting the stage for progressive glomerulosclerosis regardless of the initial cause. Angiotensin II also has direct proapoptotic effects on podocytes.85 Excessive protein uptake by podocytes induces podocyte TGFβ,86 which promotes apoptosis and leads to endoplasmic reticulum stress, cytoskeletal reorganization, and dedifferentiation.87 Drugs that are aimed at the inhibition of RAS (such as angiotensin-converting–enzyme [ACE] inhibitors and angiotensin-receptor blockers) lower intraglomerular filtration pressures through the inhibition of angiotensin II–mediated vasoconstriction of the efferent arteriole. ACE inhibition also augments bradykinin, which contributes to efferent arteriolar dilatation. The resulting reduction in proteinuria exerts a protective effect on podocytes and tubular cells.
PROGNOSTIC FEATURES
A variety of clinical and pathologic features predict outcome. Black race, increased degrees of proteinuria and renal insufficiency, and increased severity of interstitial fibrosis and tubular atrophy in biopsy specimens are associated with a worse outcome. Patients who have a partial or complete remission of proteinuria have better outcomes than those who do not.88 The histologic variant also correlated with remission status and outcome in two large case series,53,54 in which rates of complete and partial remission were highest for the tip variant, were intermediate for the cellular, perihilar, and NOS variants, and were lowest for the collapsing variant. Renal survival was inversely related to remission status, with the best rates of renal survival in the tip variant and the worst rates in the collapsing variant.53,54 The prognosis in the adaptive form of the disease is typically much better than in the primary form, possibly as a consequence of an increased likelihood of complete or partial remission with RAS inhibition in this population.
THERAPY
The goal of therapy is to induce a complete or partial remission of proteinuria and preserve renal function. Even partial remission is associated with improved long-term survival.89,90 The treatment of primary focal segmental glomerulosclerosis is empiric and based on the rationale that the permeability factor derives from a dysregulated immune response. In addition, these therapies have beneficial effects directly on podocytes.91
Children with the nephrotic syndrome are treated empirically with oral prednisone (60 mg per square meter of body surface per day) for 4 to 6 weeks, a regimen that is based on the statistical likelihood that most children (approximately 80%) will have glucocorticoid-responsive minimal change disease. In most centers, usually only children with glucocorticoid resistance are subjected to renal biopsy. In contrast, adults with the nephrotic syndrome usually undergo renal biopsy before the initiation of therapy, since the possible causes are far more varied.
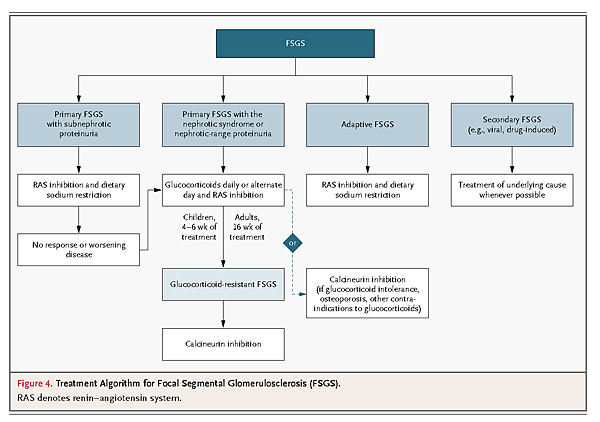
Once a diagnosis is established on biopsy, potential secondary causes that require specific therapies should be ruled out before a patient is presumed to have primary focal segmental glomerulosclerosis. For example, the form of the disease that is caused by HIV-1 infection is treated with antiretroviral therapy, and drug-induced forms are managed by discontinuation of the inciting agent. Patients with focal segmental glomerulosclerosis receive RAS blockade and dietary sodium restriction as initial therapy. In the adaptive form of the disease, such therapy typically results in the diminution of proteinuria to less than 1 g per day. There is no evidence to support glucocorticoid therapy in adaptive or genetic forms of the disease. Some genetic forms may respond to empirical therapy with calcineurin inhibitors.
An algorithm for the treatment of primary focal segmental glomerulosclerosis is illustrated in Figure4
High-dose glucocorticoid therapy can be given as 1 mg per kilogram of body weight daily or as 2 mg per kilogram on alternate days. In adults, a response to glucocorticoids may take up to 16 weeks,92 after which the drugs can be slowly tapered over a period of 3 to 6 months. There is little evidence to recommend glucocorticoid therapy in patients with the primary form of the disease that is not accompanied by the nephrotic syndrome. Therapy for glucocorticoid-resistant focal segmental glomerulosclerosis is a calcineurin inhibitor, either cyclosporine93 or tacrolimus. In some patients, such as those with diabetes, a psychiatric disorder, or severe osteoporosis, concern about the side effects of glucocorticoid therapy may prompt the selection of a calcineurin inhibitor alone as first-line therapy. Cyclosporine can be given in divided doses of 3 to 5 mg per kilogram per day for 4 to 6 months to induce remission. Patients are more likely to remain in remission if calcineurin inhibitor therapy is continued for at least 12 months before slowly tapering. In addition to the systemic immunosuppressive properties of glucocorticoids and calcineurin inhibitors, these drugs exert direct effects on the podocyte that enhance prosurvival pathways and stabilize the actin cytoskeleton.91,94 The control of blood pressure and hyperlipidemia is also a critical element of supportive care.
A randomized trial was conducted in glucocorticoid-resistant children and adults up to 40 years of age comparing a 12-month course of cyclosporine therapy with a combination of oral pulse dexamethasone and mycophenolate mofetil.95 Partial or complete remission occurred in 46% of the cyclosporine group versus 33% of the group receiving dexamethasone–mycophenolate mofetil, a difference that was not statistically significant. Although somewhat underpowered, this study suggests that these regimens have limited additional benefit in glucocorticoid-resistant patients and shows the potential for toxicity from large doses of glucocorticoids.
Glucocorticoid and calcineurin inhibitor therapies are successful in approximately 50% of patients. Other therapies have been tried, including alkylating agents, plasmapheresis, and even the anti–B-cell monoclonal antibody rituximab, which also stabilizes the podocyte actin cytoskeleton,96 but none of these therapies have been shown to be effective. Sirolimus has been associated with adverse events, including acute renal failure.97
Most patients with progressive focal segmental glomerulosclerosis have persistent nephrotic-range proteinuria. Although patients with non-nephrotic proteinuria are at a reduced risk for progression to end-stage renal disease, sustained non-nephrotic proteinuria is associated with an increased risk of death and complications from cardiovascular causes.98 Thus, the control of hypertension, hyperlipidemia, and edema is important in risk management.
RECURRENCE AFTER KIDNEY TRANSPLANTATION
In approximately 40% of patients with primary focal segmental glomerulosclerosis with end-stage renal disease who undergo kidney transplantation, recurrent disease develops in the allograft. Risk factors for recurrence include younger age (especially in children 6 to 15 years of age), nonblack race, a rapid course to end-stage renal disease (<3 years) in the native kidney, heavy proteinuria in the period before transplantation, and the loss of previous allografts to recurrence.99 Early recurrent focal segmental glomerulosclerosis resembles minimal change disease with extensive foot-process effacement, but repeat biopsy samples show evolution to lesions associated with focal segmental glomerulosclerosis over time. In such cases, the histologic subtype is the same as that in the native kidney in approximately 80% of patients, supporting the persistence of a similar pathogenesis.100 Plasmapheresis to remove the putative permeability factor is most beneficial early in the course of recurrence and is reported to lead to remission after 8 to 12 treatments.99
CONCLUSIONS
Focal segmental glomerulosclerosis is a common pattern of glomerular disease comprising diverse clinical and pathologic syndromes. All forms of the disease share podocyte injury and depletion as central mediators of the pathology. Great progress has been made in unraveling the pathogenesis of genetic and secondary forms. Advances in identification of the permeability factors causing the common primary form hold promise for the design of more targeted therapies.











 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 