Ismail Cinel, MD, PhD; Steven M. Opal, MD
Abstract and Introduction
Abstract
Background: Remarkable progress has been made during the last decade in defining the molecular mechanisms that underlie septic shock. This rapidly expanding field is leading to new therapeutic opportunities in the management of severe sepsis.
Aim: To provide the clinician with a timely summary of the molecular biology of sepsis and to better understand recent advances in sepsis research.
Data Selection: Medline search of relevant publications in basic mechanisms of sepsis/severe sepsis/septic shock, and selected literature review of other manuscripts about the signalosome, inflammasome, apoptosis, or mechanisms of shock.
Data Synthesis and Findings: The identification of the toll-like receptors and the associated concept of innate immunity based upon pathogen- or damage-associated molecular pattern molecules allowed significant advances in our understanding of the pathophysiology of sepsis. The essential elements of the inflammasome and signal transduction networks responsible for activation of the host response have now been characterized. Apoptosis, mitochondrial dysfunction, sepsis-related immunosuppression, late mediators of systemic inflammation, control mechanisms for coagulation, and reprogramming of immune response genes all have critical roles in the development of sepsis.
Conclusions: Many of these basic discoveries have direct implications for the clinical management of sepsis. The translation of these bench-to-bedside findings into new therapeutic strategies is already underway. This brief review provides the clinician with a primer into the basic mechanisms responsible for the molecular biology of sepsis, severe sepsis, and septic shock.
Introduction
In sepsis, the expected and appropriate inflammatory response to an infectious process becomes amplified leading to organ dysfunction or risk for secondary infection. A continuum exists from a low grade systemic response associated with a self-limited infection to a marked systemic response with solitary or multiorgan dysfunction, i.e., severe sepsis. As a clinical syndrome, sepsis occurs when an infection is associated with the systemic inflammatory response.
The complex toll-like receptor signaling and associated downstream regulators of immune cell functions play a crucial role in the innate system as a first line of defense against pathogens.[1] However, signaling is sometimes conflicting and a sustained inflammatory response can result in tissue damage. In addition, a reduction in their antimicrobial capacity may set the stage for opportunistic and/or super infections.[2] Severe sepsis may also be associated with an exaggerated procoagulant state. This may lead to ischemic cell injury, an effect that further amplifies the damage caused by inappropriate inflammation. A microvasculature injured by inflammation and ischemia, in turn, further deranges the host response by altering leukocyte trafficking, generating apoptotic microparticles, and increasing cellular hypoxia.[3] Mitochondrial dysfunction, an acquired intrinsic defect in cellular respiration termed cytopathic hypoxia, also has an important role by decreasing cellular oxygen consumption in this chaotic process. In this review, we highlight the current understanding of the basic molecular mechanisms that modulate these events so as to produce sepsis.
Pattern Recognition Receptors, Pathogen-Associated Molecular Patterns (PAMPs) and Danger-Associated Molecular Patterns (DAMPs)
The initiation of the host response during sepsis or tissue injury involves three families of pattern recognition receptors (PRRs): 1) toll-like receptors (TLRs); 2) nucleotide-oligomerization domain leucine-rich repeat (NOD-LRR) proteins; and 3) cytoplasmic caspase activation and recruiting domain helicases such as retinoic-acid-inducible gene I (RIG-I)-like helicases (RLHs).[4,5] These receptors initiate the innate immune response and regulate the adaptive immune response to infection or tissue injury.
Gram-positive and Gram-negative bacteria, viruses, parasites, and fungi all possess a limited number of unique cellular constituents not found in vertebrate animals. These elements are now referred to as PAMPs, or more appropriately microbial-associated molecular patterns, as these molecules are also common in nonpathogenic and commensal bacteria.[6] PAMPs bind to PRRs, such as TLRs, expressed on the surface of host cells. Cytoplasmic PRRs exist to detect invasive intracellular pathogens.[7] The NOD proteins recognize common fragments of bacterial peptidoglycan. Diamino-pimelate from Gram-negative bacteria is the ligand for NOD1 and muramyl dipeptide from peptidoglycan is the ligand for NOD2 in the cytosol (see Figs. 1 and 2). The PRRs also recognized damage signals from the release of endogenous peptides and glycosaminoglycans from apoptotic or necrotic host cells[8-10]Caspase activation and recruiting domain helicases primarily recognize viral nucleic acids and activate antiviral measures including the type I interferons.
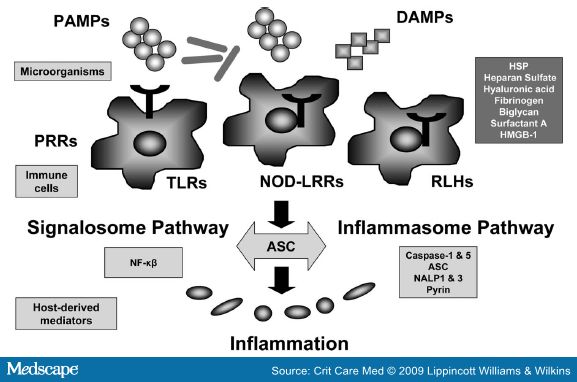
Figure 1.
Figure 2.
Inflammasome and Signalosome Pathways
TLRs induce pro-interleukin(IL)-1beta production and prime NLR-containing multiprotein complexes, termed inflammasomes, to respond to bacterial products and products of damaged cells.[17,19]This results in caspase-1 activation and the subsequent processing of pro-IL-1β to its active extracellular form IL-1β.[19] Caspases are a set of cysteine proteases that alter the enzymatic activity of target proteins at specific peptide sequences adjacent to aspartate moieties. Caspases are important in process of apoptosis, cellular regulation, and inflammation (Figs. 1 and 3). One of the many targets of the caspase cascade is caspase activated DNase (CAD). CAD activation induces DNA fragmentation characteristic of programmed cell death (apoptosis).
Figure 3.
The posttranslational activation of caspase-1 is tightly regulated by inflammasome, the known components of which include caspase-1, ASC (apoptosis-associated speck-like protein containing a caspase activation and recruiting domain), NALP1 (NACHT, leucine rich repeat and pyrin domain containing 1), and caspase-5.[20]Additionally, alternate inflammasome constructions have been suggested to contain pyrin, NALP3, and other members of the NOD-LRR family.[21-23] ASC facilitates inflammasome assembly thus triggering caspase-1 activation and IL-1β processing. Interestingly, ASC can also regulate the nuclear factor (NF)-κB pathway, thus linking the inflammasome to the signalosome (Fig. 1).[24]
The inflammasome pathways contribute to the inflammatory response in sepsis.[25] Caspase-1 knock out mice are protected from sepsis[26] while a naturally occurring polymorphism for human caspase-12, a putative regulator of caspase-1, has been linked to sepsis.[27,28] Thus, caspase-1 activation appears to be a prerequisite for a competent immune response.[29] Recently, the inflammasome components have been shown to be significantly lower in septic shock patients during the early stages of systemic inflammatory response with elevated plasma cytokines levels.[30] This monocyte deactivation process may be maladaptive in the later phases of sepsis and predispose to secondary infection.
Caspase-1 and its proinflammatory cytokine products are likely to contribute to the pathogenesis of sepsis in overwhelming inflammation. However, like many other essential elements of innate immunity, caspase-1 also has a positive impact on host defense against several infections up-regulating microbial killing mechanisms such as the production of reactive oxygen and nitrogen species (ROS and RNS).[31,32] Negative regulation of TLRs and TLR-induced programmed cell death has been defined.[7] Apoptosis induction is used by Pseudomonas aeruginosa to inhibit the secretion of immune and proinflammatory mediators by target cells.[33]
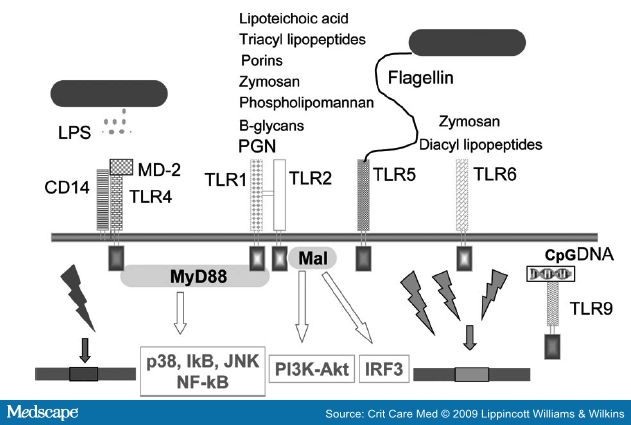
MyD88 protein and IL-1 receptor-associated kinase pathways activate NF-κB during the innate immune response.[34] NF-κB is a transcription factor that is constituted by homo- or heterodimers of the Rel protein family with a pivotal role in inflammation, cell survival, and proliferation. In unstimulated cells, NF-κB is maintained in a latent form in the cytoplasm by means of sequestration by inhibitory κB (IκB) proteins. NF-κB activating stimuli, such as cytokines, viruses, and lipopolysaccharide (LPS), induce the degradation of inhibitory κBs by the proteasome, unmasking the nuclear localization signal of NF-κB, resulting in its nuclear translocation, binding to NF-κB motifs, and gene transcription. Dynamic redox control of NF- κB through glutaredoxin-regulated S-glutathionylation of inhibitory κBs has been demonstrated.[35] NF-κB, subject to regulation by redox changes, has been shown to be involved in the transcriptional regulation of more than 150 genes with a significant portion demonstrating proinflammatory properties.[36] NF-κB is readily activated upon intraperitoneally LPS challenge within 4 hrs in lung, liver, and spleen.[37] A variant IL-1 receptor-associated kinase -1 haplotype has recently been demonstrated to affect the magnitude of NF-κB activation and directly correlates with an increased incidence of septic shock and significantly reduced survival rates.[38] The impact of NF-κB signaling is tissue-specific as deficient NF-κB activation in intestinal epithelium is associated with increased inflammation in vivo.[39,40] Both studies demonstrate that defects of NF-κB signaling cause immunosuppression which triggers and maintains inflammation. Thus, it can be suggested that inhibition of massive NF-κB activation in vivo leads to reduced inflammatory responses, at least during certain phases and certain tissues (i.e., parenchymal tissues) in sepsis. On the other hand, this activation is followed by negative NF-κB regulation favoring apoptosis in immune cells which may lead to immunosuppression and fatal outcome in severe sepsis. Recently, using specific gene-targeted deletions, it has been shown that deletion of MyD88 caused a worsened survival in a model of severe peritonitis, despite the marked decrease in sepsis-induced T and B lymphocyte apoptosis suggesting MyD88, like NF-κB, is also critical host survival in sepsis.[41]
Toll-Like Receptor Signaling
The Role of Phosphoinositide 3-Kinase
Phosphoinositide 3-kinase (PI3K), a signal transduction enzyme, and the downstream serine/threonine kinase Akt (also known as protein kinase B) have been reported in cellular activation, inflammatory responses, chemotaxis, and apoptosis (Figs. 2 and 3).[42] PI3K can function either as a positive or negative regulator of TLR signaling. PI3Ks (three types) act at several steps downstream of TLRs, depending on the cell type and/or the engagement of a specific TLR regulating downstream signaling to NF-KB transactivation or to mitogen-activated phosphokinase activation.[43,44] As a positive mediator of TLR signaling, PI3K together with p38 and extracellular regulated kinase (ERK)1/2 mitogen-activated phosphokinases, lead to production of proinflammatory cytokines IL-1β, IL-6, and IL-8 upon microbial challenge.[44,45] In detail, PI3K activation appeared to have a significantly promoting function for these mediators in monocytes, whereas activation appeared to limit the LPS response for generation of these cytokines in neutrophils.[46] However, PI3K inhibition resulted in impaired oxidative burst and phagocytosis activity in both neutrophils and monocytes. By limiting C5a-mediated effects on neutrophil cytokine generation, and promoting oxidative burst and phagocytosis, PI3K activation seems to be a therapeutic approach for limiting inflammation in sepsis.
On the other hand, PI3K/Akt signaling pathway acts as an endogenous negative feedback mechanism that serves to limit proinflammatory and apoptotic events as seen in monocytes in response to endotoxin.[47] The suppressive role in inflammation and coagulation was confirmed in mouse model of endotoxemia.[48] It can also promote the generation of anti-inflammatory cytokine IL-10.[49] PI3K/Akt has an effect in balancing Th1 vs. Th2 responses.[50]Overexpression of Akt in lymphocytes decreases lymphocyte apoptosis, a Th1 cytokine propensity, and improve outcome in cecal ligation and puncture-induced sepsis.[51] Peptide-mediated activation of Akt and extracellular regulated kinase signaling protects lymphocytes from numerous apoptotic stimuli both in vitro and in vivo.[52]This suggests again the survival advantage of PI3K/Akt pathway activation for the later stages of sepsis.
The Role of Rho GTPases
The Rho family of small GTPases is one of the master regulators of cell motility, as they control actin cytoskeleton remodeling. RhoA, Rac1, and CDc42 are the well known family members which act molecular switches regulating responses of innate immune cells related to pathogen sensing, intracellular uptake, and destruction.[53] These GTPases play both unique and overlapping roles in phagocyte functions including migration, chemotaxis, and optimal bacterial killing.[54] It has been shown that PAMPs may utilize GTPases through TLRs (i.e., TLR-2, -4, -3, and -9) in which Rac1 activation is required for PI3K activation upon TLR2 stimulation.[55] RhoA regulates not only cytoskeletal events, which mediate neutrophil migration, but also contributes to NF-κβ-dependent proinflammatory gene transcription (Fig. 3).[56] It has been suggested that ROCK inhibition could attenuate cytoskeletal rearrangement of endothelial cells, leading to decreased neutrophil emigration into the lung parenchyma in LPS-induced lung injury.[57] An important role for rho kinase in leukocyte recruitment is also supported in an endotoxemic liver injury model.[58] The recently emerged connections between TLR signaling and small Rho-GTPases seem to provide new therapeutic avenue of research in sepsis.
Toll-Like Receptor Signaling and Regulatory T Cells (Tregs)
To balance self-tolerance and immunity against pathogens, the immune system depends on both up-regulatory and down-regulatory mechanisms. Recent studies have suggested that several lymphocyte subpopulations (i.e., CD4+CD25+ Foxp3+ T regulatory-cell [Tregs]) may have the capacity to actively suppress an adaptive immune response and may potentially be involved in septic immune dysfunction. Naturally occurring Tregs express the transcription factor forkhead box protein (FoxP3), which is induced by the anti-inflammatory cytokine transforming growth factor-β.[59,60] TLR triggering induces dendritic cell maturation also, which is essential for the induction of adaptive immune responses.[61] Studies have recently highlightened the importance of TLRs on Tregs.[62] In this regard, the dominant role of TLR2 signaling on the Treg-mediated immune suppression has been demonstrated.[63,64]
Tregs control inflammatory reactions to commensal bacteria and opportunist pathogens and play a major role in suppressing immune reactivity, ranging from autoimmunity to infectious disease[65] and to injury.[66] Monneret et al[67] observed that sepsis increases CD4+CD25+ T cells in the peripheral blood of septic patients. This was subsequently found to be a relative increase in Tregs due to a decrease in the CD4+CD25- T effector cell populations.[68]Furthermore, Treg-mediated induction of alternatively activated macrophages has been demonstrated suggesting as one of the causes of immune dysfunction in systemic inflammatory response syndrome and sepsis.[69,70] Targeting apoptosis of Tregs may be a new therapeutic approach in preventing the continuum of sepsis to severe sepsis. However, it has been reported that depletion of CD25+ cells before inducing sepsis did not alter septic mortality pointing the need of more studies to clarify the significance of this cell population's expansion in sepsis morbidity.[71,72]
Adoptive transfer of Tregs before or following the initiation of polymicrobial sepsis improved survival by enhancing tumor necrosis factor (TNF)-α production and bacterial clearance.[73] Tregs inhibit LPS-induced monocyte survival through the Fas/FasL dependent proapoptotic mechanism, which might play a role in the resolution of exaggerated inflammation.[74] The positive and negative effects of TLR on Tregs are curious in sepsis, and the dynamics of TLR expression on immune-suppressive Tregs upon inflammation or in relation to type of pathogen are needed to illuminate.
Neutrophils and Monocyte/Macrophages in Inflammation
Myeloid cells including neutrophils and elements of monocyte/macrophage lineage are heavily armed with large stores of proteolytic enzymes and with the capacity to rapidly generate ROS and RNS to degrade internalized pathogens. The highly proapoptotic nature of neutrophils is designed to maintain a balance between antimicrobial effectiveness and the potential for neutrophil-associated damage to the host in septic challenge or in other injurious processes, such as trauma or ischemia/reperfusion.[75]Host tissue damage in severe sepsis may arise via a variety of mechanisms including premature neutrophil activation during migration, extracellular release of cytotoxic molecules and toxins during microbial killing, removal of infected or damaged host cells or debris during host tissue remodeling, and failure to terminate acute inflammatory responses.[76] Therefore, to maximize host defense capabilities while minimizing damage to host tissues, neutrophil microbial responses are tightly regulated. On the other hand, quorum sensing (the ability of bacteria to assess their population density) has been found to have a crucial role in regulating tissue invasion by bacterial pathogens, and inhibitors of quorum sensing system provide new avenues for intervention against invasive pathogens.[1]Evidence now exists that quorum sensing system can even open up bidirectional lines of communication between bacteria and the human host.
Leukocyte-endothelial interactions, which may also contribute to inflammation-mediated injury, involve two sets of adhesion molecules, selectins, and integrins.[77,78] P-selectin is expressed on platelets; E- and P-selectins are expressed by endothelial cells, whereas L-selectin is expressed on leukocytes. Selectins mediate neutrophil rolling along activated endothelial surfaces as circulating neutrophils decelerate to engage endothelial receptors. The beta-2 intergrins (CD11/CD18 complexes) mediate tight adhesion to endothelial membranes, allowing subsequent egress of neutrophils to extravascular sites of inflammation.[78]Activated neutrophils stimulate transendothelial albumin transport through intracellular adhesion molecule-1 mediated, Scr-dependent caveolin phosphorylation. The role of caveolin in inflammatory response in sepsis has been recently defined (Fig. 3).[79-82]
Neutrophils contribute to blood coagulation in localized inflammation and in generalized sepsis.[1,3,75] During systemic inflammation, homeostatic mechanisms are compromised in the microcirculation including endothelial hyperactivity, fibrin deposition, microvascular occlusion, and cellular exudates that further impede adequate tissue oxygenation. Neutrophils participate in these rheologic changes through their augmented binding to blood vessel walls and through the formation of platelet-leukocyte aggregates.[83] Neutrophil elastase, other proteases, glycases and inflammatory cytokines degrade endogenous anticoagulant activity, and impair fibrinolysis on endothelial surfaces favoring a procoagulant state.[1]
The Role of Apoptosis
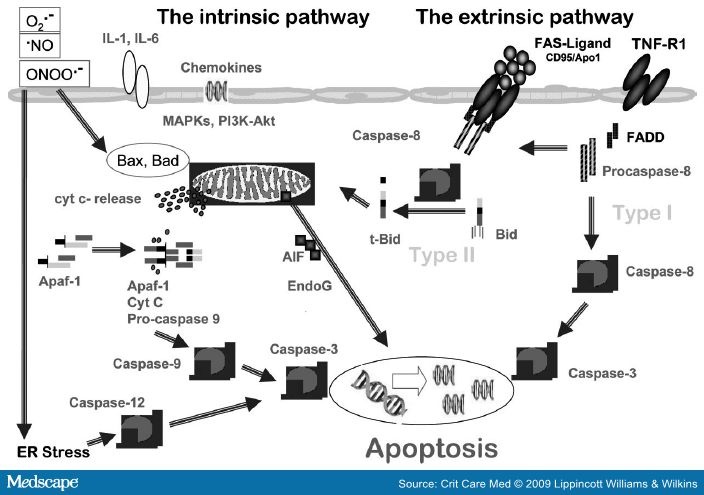
Sepsis-induced neutrophil-mediated tissue injury has been demonstrated in a variety of organs including the lungs,[84-86] diaphragm,[87] kidneys,[86] intestine,[88,89] and liver.[86] Apoptosis is a counter-regulator of the initial inflammatory response in sepsis.[90] Neutrophils are constitutively proapoptotic and apoptosis is fundamental for the resolution of inflammation and cell turnover. Neutrophils can undergo apoptosis via intrinsic and extrinsic pathways; the latter also requires mitochondrial amplification (Fig. 4).[91] The role played by mitochondria in the regulation of neutrophil life span is more crucial than in other cell types in the body.[92] As neutrophils kill pathogens using ROS and RNS and a mixture of lytic enzymes, delayed clearance of neutrophils in sepsis can potentially contribute to cell/organ injury. Importantly, the phagocytosis of bacteria and fungi accelerates neutrophil apoptosis. Apoptotic cell clearance induces anti-inflammatory effects in tissues. It has been shown that intratracheal administration of killed E. coli attenuated lung injury and improved survival in an intestinal ischemia/reperfusion injury associated with marked pulmonary neutrophil infiltration.[93]
Figure 4.
Cytokine-induced prolonged neutrophil survival is accompanied by evidence of increased neutrophil activation, including augmented respiratory burst activity.[90] Neutrophils from patients with sepsis manifest markedly prolonged survival in vitro in association with evidence of cellular activation.[94] Phagocytosis of apoptotic neutrophils by macrophages inhibits the release of proinflammatory cytokines and promotes the secretion of anti-inflammatory cytokines.[95] In contrast, inefficient apoptotic cell clearance is proinflammatory and immunogenic.[96] The recognition of apoptotic cells by macrophages is largely dependent on the cell surface appearance of an anionic phospholipid, phosphatidylserine (PS), which is normally confined to the inner leaflet of the plasma membrane.[97] Asymmetric distribution of PS across the plasma membrane is mainly because of the activity of a specialized enzymatic mechanism, aminophospholipid translocase. S-nitrosylation of critical cysteine residues inhibits aminophospholipid translocase, leading to PS externalization. PS expression during apoptosis generates an eat-me signal (Fig. 5), which in turn triggers clearance of apoptotic cells and suppresses the inflammatory response.[98] It has been demonstrated that S-nitrosylation of critical cysteine residues in aminophospholipid translocase using a cell-permeable transnitrosylating agent, S-nitroso-acetyl- cysteine, resulted in egression of PS to the outer surface of the plasma membrane, rendering these cells recognizable by macrophages.[99] The therapeutic potential of regulating neutrophil life-span in sepsis remains to be determined. Care must be exercised in regulating of this pathway, as sepsis enhances the capacity of macrophages to clear expanded apoptotic populations, a mechanism contributing to septic immune suppression.[100]
Figure 5.
The Role of ROS and RNS
ROS and RNS exert several beneficial physiologic functions, such as intracellular signaling for several cytokines and growth factors, second messengers for hormones and redox regulation. Despite their importance as a defense mechanism against invading pathogens, an overwhelming production of ROS and RNS or a deficit in oxidant scavenger and antioxidant defenses result in oxidative/nitrosative stress, a key element in the deleterious processes in sepsis (Fig. 6).[101,102]
Figure 6.
Stimulated neutrophils produce ROS and RNS through the nicotinamide adenine dinucleotide phosphate oxidase complex, myeloperoxidase and xanthine oxidoreductase and represent a defense mechanism against invading microorganisms.[103] Lipopolysaccharide and other proinflammatory mediators activate nicotinamide adenine dinucleotide phosphate oxidase to produce superoxide radical (O2 -). In aqueous environments, superoxide radical is rapidly catalyzed by superoxide dismutase hydrogen peroxide (H2O2) and hydroxyl radicals. Myeloperoxidase from neutrophil azurophilic granules produces hypochlorous acid from hydrogen peroxide (H2O2) and chloride anion (Cl-) during respiratory burst. These radicals are highly cytotoxic, and neutrophils used them to kill bacteria and other pathogens. Expression of human xanthine oxidoreductase is markedly up-regulated by hypoxia, ischemia/reperfusion, LPS, and TNF-α. Increased activity of xanthine oxide, one the important contributors of ROS production, has been reported in adult and pediatric patients with sepsis.[104]
O2 - in the presence of nitric oxide, generates peroxynitrite (ONOO-), a key player in the pathogenesis of sepsis-induced organ dysfunction. ONOO- can cause DNA strand breakage, which triggers the activation of DNA repair enzymes such as poly (adenine dinucleotide phosphate-ribose) polymerase (Fig. 3). Poly (adenine dinucleotide phosphate-ribose) polymerase inhibitors protect against oxidative and nitrosative stress-induced organ dysfunction in endotoxemia.[87-89] Recently, the potential role of poly (adenine dinucleotide phosphate-ribose) polymerase activation has been implicated in the pathogenesis of myocardial contractile dysfunction associated with human septic shock.[105]
Novel Cytokines in Inflammation
High Mobility Group Box-1 and Receptor for Advanced Glycation End-Products
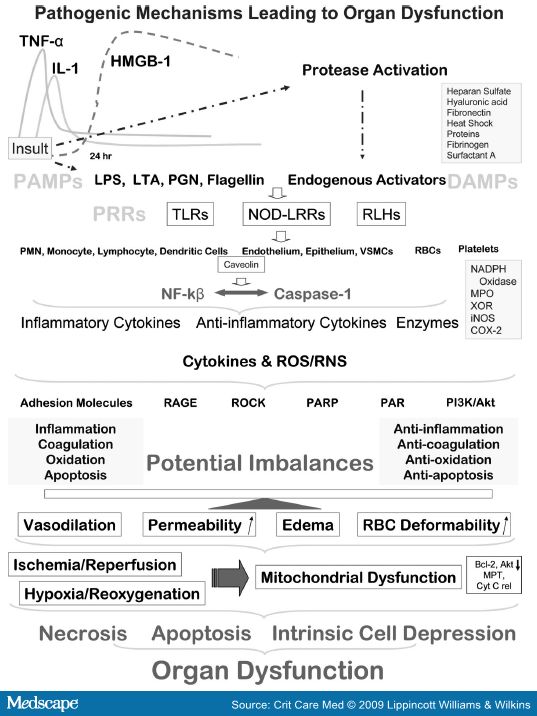
High mobility group box-1 (HMGB-1) is a nonhistone, nuclear DNA-binding protein involved in nucleosome stabilization and gene transcription. However, when HMGB-1 is released in large quantities into the extracellular environment, it becomes a lethal mediator of systemic inflammation (Fig. 3).[106] Indeed, it has been recently shown that it has a weak proinflammatory activity by itself and binding to bacterial substances including lipid molecules such as phophatidylserine strengthens its effects.[107] Interestingly, phosphatidylserine has been implicated in the regulation of inflammation and HMGB-1 might thus regulate its anti-inflammatory activities.[108]
HMGB-1 is released into the extracellular space through acetylation or phosphorylation.[109,110] HMGB-1 is either passively released from necrotic cells, and a mechanism that represents a process adopted by the innate immune system to recognize damaged and necrotic cells, or actively secreted by immune cells including macrophages and neutrophils to trigger inflammation.[106] Recently, HMGB-1 release from macrophages has been shown during the course of apoptosis as well as necrosis and defined as a downstream event of cell apoptosis during severe sepsis.[111,112]After treatment with LPS or various cytokines such as TNF-α, IL-1β, or IFN-γ, HMGB-1 is released from activated macrophages within 4 hrs and reaches a plateau around 18-24 hrs. It binds to several transmembrane receptors such as receptor for advanced glycation end products (RAGE), TLR-2, and -4, activating NF-κB and extracellular regulated kinase 1/2.[113,114]
Although HMGB-1 was originally described as a late mediator of endotoxin-induced lethality,[115] recent studies indicate a role for HMGB-1 in angiogenesis, tissue repair, and regeneration.[116] HMGB-1 appears to be a novel myocardial depressant factor upon release by resident myocardial cells following tissue injury. HMGB-1 might decrease energy utilization in ischemic tissue, thereby preventing injured myocytes from worsening ATP depletion that eventuate in necrosis.[117] On other hand, excessive release of HMGB-1 in sepsis might contribute to sustained inflammation and to profound myocardial depression.
The cholinergic anti-inflammatory pathway is a neural mechanism that inhibits the expression of HMGB-1 and other cytokines.[118-121] Signals transmitted via the vagus nerve, the principal nerve of the parasympathetic nervous system, significantly attenuate the release of HMGB-1 and other cytokines in inflammation in animal and human studies.[122]
The remarkable binding characteristics of HMGB-1 suggest another important role for this protein in the extracellular fluid. HMGB-1 might serve as a shuttle platform for LPS and other microbial mediators for docking to CD14 and recognition by the TLRs[108,123] or through binding to host-derived proinflammatory mediators, such as IL-1beta.[124]
Elevated levels of HMGB-1 are measurable in the majority of patients up to 1 wk after the diagnosis of sepsis or septic shock and are correlated with the degree of organ dysfunction.[125,126] However, serum HMGB-1 levels do not consistently identify nonsurvivors from survivors as a predictor of hospital mortality.[127] As HMGB-1 is late inflammatory cytokine of sepsis, it provides a wide therapeutic time window for clinical intervention and remains an attractive target for sepsis treatment.
RAGE, a member of the immunoglobulin superfamily, is a pattern-recognition receptor that binds diverse classes of endogenous molecules including HMGB-1. It has been defined as part of a newly appreciated component of the innate immune system referred to as the danger associated molecular pattern system (Fig. 3).[128] Membrane bound and soluble forms of RAGE (sRAGE) have been detected in plasma. Soluble RAGE, anti-RAGE antibody (Fab 2 fragment) or data from RAGE-/- animals have been shown to decrease inflammation, reduce neutrophil extravasation, and reduce migration.[129] Recently, the demonstration of survival benefit after delayed administration of anti-RAGE antibody in a murine model of polymicrobial sepsis, even when delayed up to 24 hrs, provides a therapeutic rationale for the use of anti-RAGE mAb as a salvage therapy for established severe sepsis.[130]
Macrophage Migration Inhibitory Factor
Migration inhibitory factor (MIF) acts as a stress response mediator and proinflammatory cytokine upon induction by glucocorticoids.[131] This protein is readily measurable in patients with sepsis, and MIF probably contributes to the pathogenesis of sepsis. Inhibition of MIF or its targeted deletion attenuates TNF-α and IL-1β expression and protects mice from lethality is experimental sepsis.[132,133] Systemic challenge of animals with MIF increases LPS-related lethality.[133] MIF promotes the expression of TLR4 on macrophages, and thereby sensitizes these immune effector cells to LPS.[134]
The immunoregulatory effects of MIF might be crucial to the control and resolution of the inflammatory response as a consequence of its ability to regulate activation-induced apoptosis.[135] High MIF levels delay the removal of activated monocytes/macrophages by apoptosis. This prolongs monocyte/macrophage survival, increases cytokine production, and sustains an ongoing proinflammatory response.
Mitochondrial Dysfunction in Inflammation
Although microvascular flow abnormalities occur, findings of decreased oxygen consumption[136] and elevated tissue oxygen tension,[137] yet minimal cell death despite functional and biochemical derangements,[138] suggest that the problem lies more in cellular oxygen utilization rather than a problem with oxygen delivery.[139] It is postulated that prolonged and systemic inflammatory insult is accompanied by a basic tissue survival response mediated by switching off its energy-consuming biophysiological processes. Recent evidence suggests that sepsis and septic shock severely impair the mitochondria,[140,141] and the severity and outcome of organ dysfunction could be related to mitochondrial dysfunction.[142] Depleted levels of reduced glutathione, an important intramitochondrial antioxidant, in combination with excess generation of ROS and RNS severely inhibit oxidative phosphorylation and ATP generation.[142] This acquired intrinsic derangement in cellular energy metabolism which has also been termed cytopathic hypoxia, contributes to reduced activities of mitochondrial electron transport chain enzyme complexes and impaired ATP biosynthesis, potentially organ dysfunction in sepsis.[141,143,144]
Sepsis-related derangements in mitochondrial function can activate the ubiquitin proteolytic pathway in skeletal muscle of septic patients.[145] Mitochondrial permeability transition pore seems to be involved in sepsis-induced mitochondrial damage, since its inhibition significantly improved organ function and reduced mortality in rodents.[146] Whether sepsis-related reduction in energy supply could result in a state of cellular shutdown analogous to myocardial stunning (or hibernation) following coronary occlusion, allowing for eventual restoration of organ function and survival, has not yet been determined.
Impact of Inflammation on Coagulation
The clotting system is almost invariably activated by systemic microbial invasion.[147] Clotting is one most prominent features of sepsis. Coagulation contributes significantly to the outcome in sepsis with concurrent down-regulation of anticoagulant systems and fibrinolysis (Fig. 7). Inflammation-induced coagulation in turn contributes to further inflammation.[148] Indeed, collaboration between clotting and inflammation accounts for the basic survival strategy of walling off the damaged and infected tissues from the rest of the host.[149] The key determinant of survival in sepsis is to limit excess systemic inflammatory and coagulopathic damage while retaining the benefits of controlled antimicrobial clearance and localized clot formation.[147,150]
Figure 7.
The inflammatory reaction to tissue injury activates the clotting system, and coagulation promotes inflammation.[3,151,152] The role of procoagulant apoptotic microparticles have also been demonstrated in sepsis.[153-155] Linkage of coagulation enzymes with their serine protease activity with protease activated receptors (PAR 1-4) on endothelial surfaces increases P-selectin, cytokine production, and adhesion molecule expression, leading to microcirculatory dysfunction in severe sepsis.[156,157]
Activated Protein C.
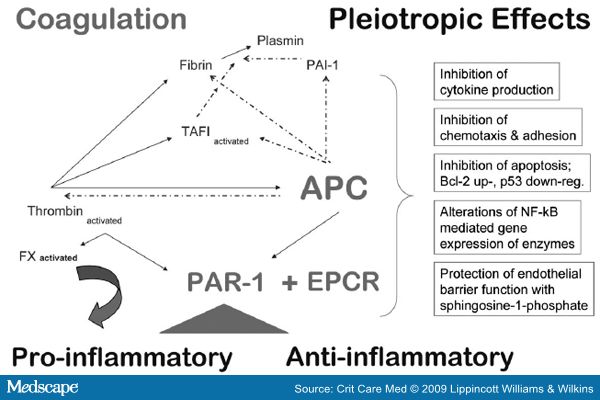
Activated protein C (APC) is derived from its zymogen protein C in contact with thrombin: thrombomodulin complexes on endothelial surfaces. Protein C was originally thought to be synthesized exclusively by the liver.[158] It has recently shown that it is strongly expressed by the endothelium and keratinocytes.[159] The conversion to APC is augmented by endothelial PC receptor (EPCR) which is present on endothelial cells, neutrophils, monocytes, and keratinocytes[160,161] whereas soluble EPCR inhibits APC anticoagulant activity.[162] Soluble EPCR is released constitutively and levels increase in patients with Gram-negative sepsis.[163] Levels of APC, protein C, and its cofactor protein S are depleted in sepsis.[164,165] Furthermore, peripheral conversion of Protein C by the thrombin: thrombomodulin complex is impaired in sepsis, further contributing to microvascular thrombosis and vascular leakage.[166]
APC is a central endogenous anticoagulant protein with antithrombotic, anti-inflammatory, antiapoptotic, and profibrinolytic activities (Fig. 8).[167,168] Although an anti-inflammatory role for APC may be an indirect consequence of its ability to reduce thrombin generation, APC also has direct anti-inflammatory properties.[169]APC can cleave and activate PAR1-dependent cellular pathways.[170] APC competes for PAR-1 binding with thrombin, but in vitro studies suggest that APC is 103- to 104-fold less potent that thrombin in cleaving PAR-1. The critical receptors required for both PC activation and APC cellular signaling (i.e., thrombomodulin, EPCR and PAR-1) are co-localized in lipid rafts on endothelial cells.[171] EPCR is associated with caveolin-1 on lipid rafts and EPCR binding to the gamma-carboxyglutamic acid domain of protein C/APC leads to its dissociation from caveolin-1 (Fig. 7). APC then engages PAR-1 generating a protective signaling pathway through coupling of PAR-1 to the pertussis toxin-sensitive G(i)-protein. Thus, when EPCR is bound by protein C, the PAR-1-dependent protective signaling responses in endothelial cells can be mediated by either thrombin or APC. These results explain how PAR-1 and EPCR participate in protective signaling events in endothelial cells.[172]
Figure 8.
Therapeutic administration of recombinant human APC (rhAPC) is currently in use as a treatment strategy for severe sepsis patients with a high risk of death.[173,174] Genetically engineered variants of APC have been designed with greater antiapoptotic activity and reduced anticoagulant activity relative to wild-type APC to increase the risk/benefit ratio of rhAPC regarding the bleeding complication.[175] A nonanticoagulant from of APC reduces mortality in experimental models of endotoxemia and sepsis.[176]
von Willebrand Factor, A Disintegrin-Like and Metalloproteinase with Thrombospondin Type-1 Motifs 13 and Ashwell Receptor
The discovery of a disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13 (ADAMTS-13) has provided new insights in pathogenesis of thrombosis in sepsis. ADAMTS-13, the principal physiologic modulator of von Willebrand factor (VWF) is produced mainly stellate cells in the liver.[177] VWF is synthesized in vascular endothelial cells and released into the plasma as unusually large VWF multimers which are rapidly degraded into smaller VWF multimers by ADAMTS-13. Deficiency of the ADAMTS-13, as observed in most forms of thrombotic thrombocytopenic purpura, increases the level of unusually large VWF multimers in plasma and leads to platelet aggregation and/or thrombus formation, especially in small arterioles, resulting in microvascular failure.[178-180]Recently, inflammation associated-ADAMTS-13 deficiency has been described in patients with systemic inflammatory response syndrome and severe sepsis (Fig. 7).[181-185] Decreased levels of ADAMTS-13 have been reported in healthy volunteers following endotoxin infusion.[186] Furthermore, reduced ADAMTS-13 levels are associated with differences in morbidity, mortality, and variables of inflammation and endothelial dysregulation in severe sepsis patients.[182,187]
The Ashwell receptor, which is the major lectin of hepatocytes, modulates VWF homeostasis. It has been recently demonstrated that the marked thrombocytopenia associated with S. pneumoniae sepsis is the result of Ashwell receptor-dependent clearance of platelets.[188] The ensuing reduction in platelet counts at the onset of sepsis protects the host against the development of disseminated intravascular coagulation. Homeostatic adaptation by this receptor moderates the onset and severity of disseminated intravascular coagulation during sepsis suggesting the improvement in host survival probability.[189] At present, it remains unclear whether blocking the Ashwell receptor may have a beneficial effect in severe sepsis.
Conclusion
A unifying concept of innate immunity is based upon pathogen-, or damage-associated molecular pattern molecules and downstream signaling pathways. This has facilitated significant advances in our understanding of the pathophysiology of sepsis and led to a multitude of clinical trials ( Table 1 ). However, further identification of the critical elements in severe sepsis that drives the transition from localized inflammation to deleterious host response remains incompletely illuminated. The limitations in our current knowledge of the molecular mechanisms in sepsis have made the design of intervention trials in clinical sepsis challenging.[190] New findings as to biomarkers and measures to detect genetic signatures of sepsis are now making their way into clinical trial designs. It is anticipated that such innovations will improve the outlook for successful development of new sepsis treatments tailored to individual patient needs.
Table 1. Ongoing and Upcoming Clinical Studies with Molecular Targets









 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 