Two medications are licensed in the United States for the treatment of infection with genital herpes simplex virus (HSV): valacyclovir (approved by the Food and Drug Administration in 1995) and famciclovir (approved in 1997). All studied and licensed drugs for HSV infections assessed to date are selective inhibitors of HSV DNA polymerase and act as analogues of nucleoside triphosphate.1 The report by Wald and colleagues in this issue of the Journal 2 introduces two fundamentally new approaches to the study of medications for the treatment of genital herpes. One approach involves the application of daily quantitative polymerase-chain-reaction (PCR) analysis to define a drug's utility in a proof-of-concept trial, and the other involves the evaluation of a novel inhibitor of HSV replication that does not target the viral DNA polymerase.
Investigators at the University of Washington have used PCR analysis to define the natural history of genital HSV infections. By enrolling highly motivated volunteers who provided samples daily, they were able to report the frequency of viral shedding and the propensity for intermittent daily shedding and were able to reproduce these findings.3,4 The findings cannot be underestimated, since they allow us to understand the variability of disease and the need for detailed virologic assessments along with clinical evaluations.
This development allows for a more efficient assessment of the value of new interventions. In the study by Wald and colleagues, the investigators used daily quantitative PCR analysis to determine the effect of a new therapeutic intervention, pritelivir. In a dose-escalating study performed over 28 days, the activity of pritelivir was shown in relation to viral shedding and the number of days of clinical lesions, with viral load as a biomarker. No evidence of resistance was detected, and there were no safety signals, although the study had limited power to detect these potential events.
Although the study was labor-intensive, it was a relatively efficient means of determining whether this new agent has some potential antiviral activity. The high compliance rates provide statistical power that could not be achieved by simply obtaining cultures three times a week or assessing the effect on clinical disease. The findings were achieved with a relatively small sample size per randomization cell (approximately 30 volunteers per group). Thus, this short-term study shows a proof of concept and allows for the design of additional studies addressing the treatment of genital HSV infections.
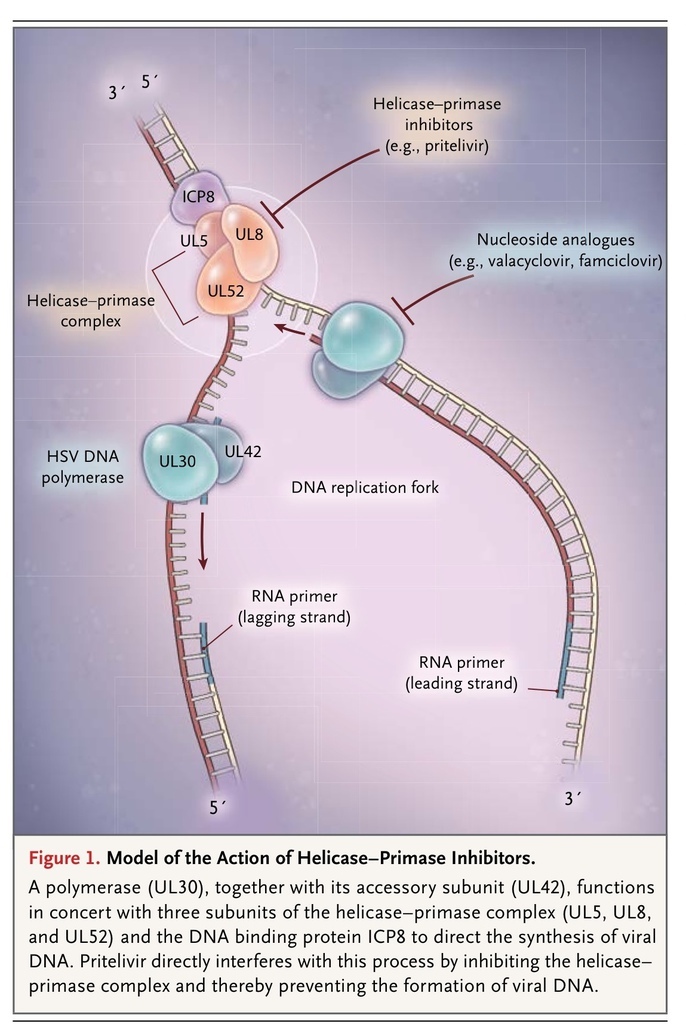
Currently approved therapies for genital herpes inhibit the synthesis of viral DNA by means of the specific inhibition of the DNA polymerase. The polymerase (UL30), together with its accessory subunit (UL42), function in concert with three subunits of the helicase–primase complex (UL5, UL8, and UL52) and ICP8 to direct the synthesis of viral DNA (Figure 1
FIGURE 1
Model of the Action of Helicase–Primase Inhibitors.
).5,6 Pritelivir interferes with this process by inhibiting the helicase–primase complex. This mechanism gives pritelivir a significant advantage because resistance to it maps to UL5; pritelivir thus remains active against viruses that are resistant to acyclovirs, typically because they harbor mutations in the thymidine kinase and DNA polymerase.1,7 Because of its unique mechanism of action, pritelivir provides an alternative to the nucleoside therapeutic agents and exhibits a distinct profile of antiviral resistance. Resistance to the nucleoside analogues is encountered regularly in the immunocompromised host, particularly in recipients of stem-cell transplants and patients in whom antiretroviral therapy for the acquired immunodeficiency syndrome has failed.
For those providing care to patients with life-threatening HSV infections,8 the potential for combination therapy may now be possible, and studies to assess this opportunity are under way. It is likely that we will soon learn more about pritelivir's ability to concentrate in the central nervous system and offer yet another alternative for combination therapy.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 