The U.S. Food and Drug Administration today approved Zerbaxa (ceftolozane/tazobactam), a new antibacterial drug product, to treat adults with complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI).

ceftolozane
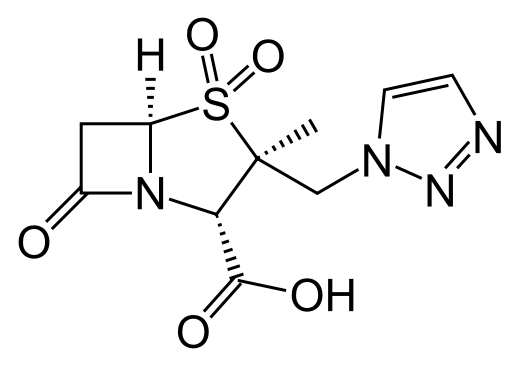
tazobactam
Zerbaxa is a combination product containing ceftolozane, a cephalosporin antibacterial drug, and tazobactam, a beta-lactamase inhibitor. Zerbaxa is used to treat cUTI, including kidney infection (pyelonephritis). It is used in combination with metronidazole to treat cIAI.
Zerbaxa is the fourth new antibacterial drug approved by the FDA this year. The agency approved Dalvance (dalbavancin) in May, Sivextro (tedizolid) in June and Orbactiv (oritavancin) in August.
“The FDA approval of several new antibacterial drugs this year demonstrates the agency’s commitment to increasing the availability of treatment options for patients and physicians,” said Edward Cox, M.D., M.P.H, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research. “We must continue to help foster the development of new antibacterial drugs and encourage prudent use of existing treatments to conserve their utility.”
Zerbaxa is the fourth new antibacterial drug product designated as a Qualified Infectious Disease Product (QIDP) to receive FDA approval. Under the Generating Antibiotic Incentives Now (GAIN) title of the FDA Safety and Innovation Act, Zerbaxa was granted QIDP designation because it is an antibacterial or antifungal human drug intended to treat a serious or life-threatening infection.
As part of its QIDP designation, Zerbaxa was given priority review, which provides an expedited review of the drug’s application. The QIDP designation also qualifies Zerbaxa for an additional five years of marketing exclusivity to be added to certain exclusivity periods already provided by the Food, Drug and Cosmetic Act.
Zerbaxa’s efficacy to treat cIAI in combination with metronidazole was established in a clinical trial with a total of 979 adults. Participants were randomly assigned to receive Zerbaxa plus metronidazole or meropenem, an FDA-approved antibacterial drug. Results showed Zerbaxa plus metronidazole was effective for the treatment of cIAI.
The efficacy of Zerbaxa to treat cUTI was established in a clinical trial where 1,068 adults were randomly assigned to receive Zerbaxa or levofloxacin, an antibacterial drug approved by the FDA to treat cUTI. Zerbaxa demonstrated it was effective in treating cUTI.
The Zerbaxa label includes a warning about decreased efficacy seen in patients with renal impairment. The most common side effects identified in the clinical trials were nausea, diarrhea, headache and fever (pyrexia).
Zerbaxa and Sivextro are marketed by Cubist Pharmaceuticals, based in Lexington, Massachusetts. Dalvance is marketed by Chicago-based Durata Therapeutics, and Orbactiv is marketed by Parsippany, New Jersey-based The Medicines Company.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 