International Journal of Surgery Volume 44, August 2017, Pages 260-268
Highlights
- •
-
In wound care, topical antiseptics may limit the potential for antibiotic resistance.
- •
-
Povidone iodine is an effective antiseptic that does not impede wound healing.
- •
-
Povidone iodine is bactericidal against Gram-positive and -negative organisms.
- •
-
No acquired bacterial resistance or cross-resistance has been reported for iodine.
- •
-
Povidone iodine aids healing in a range of acute and chronic wounds.
Abstract
Background
Of the many antimicrobial agents available, iodophore-based formulations such as povidone iodine have remained popular after decades of use for antisepsis and wound healing applications due to their favorable efficacy and tolerability. Povidone iodine's broad spectrum of activity, ability to penetrate biofilms, lack of associated resistance, anti-inflammatory properties, low cytotoxicity and good tolerability have been cited as important factors, and no negative effect on wound healing has been observed in clinical practice. Over the past few decades, numerous reports on the use of povidone iodine have been published, however, many of these studies are of differing design, endpoints, and quality. More recent data clearly supports its use in wound healing.
Methods
Based on data collected through PubMed using specified search criteria based on above topics and clinical experience of the authors, this article will review preclinical and clinical safety and efficacy data on the use of povidone iodine in wound healing and its implications for the control of infection and inflammation, together with the authors' advice for the successful treatment of acute and chronic wounds.
Results and conclusion
Povidone iodine has many characteristics that position it extraordinarily well for wound healing, including its broad antimicrobial spectrum, lack of resistance, efficacy against biofilms, good tolerability and its effect on excessive inflammation. Due to its rapid, potent, broad-spectrum antimicrobial properties, and favorable risk/benefit profile, povidone iodine is expected to remain a highly effective treatment for acute and chronic wounds in the foreseeable future.
1. Introduction
Virtually all wounds are colonized with microorganisms, often without clinical consequence and the presence of some microorganisms may even aid healing [1], [2]. However, contamination with pathogenic microbial flora can lead to infection and sepsis, which disrupts the healing continuum [3], [4]. The development of infection is determined by complex interactions between the host and microorganisms, and further influenced by the environment and therapeutic interventions [3], [5].
Although inflammation occurs as a physiological response to wounding and is required for the healing process, microbial infection can induce excessive inflammation [4]. Prolonged inflammation together with defective remodeling of the extracellular matrix and a failure of re-epithelialization are hallmark characteristics of chronic wounds [2]. Microbial colonies in chronic wounds often produce biofilms that interact with host tissue in a parasitic manner [3], [4]. The reduced metabolic state induced by biofilms can also enhance resistance to antibiotics and aid the evasion of host defense mechanisms. Biofilms are found in approximately 60% of chronic wounds and 6% of acute wounds, with eradication of the resident bacteria remaining difficult [4]. Therefore, effective antiseptics for wound healing should ideally address both inflammation and biofilm formation.
Few antimicrobial substances are generally considered for wound therapy. Of these, povidone iodine has continued to be broadly applied [7], [8], [9].
The aim of this article is to clarify the current role and use of povidone iodine in wound healing from both diverse regional and specialist's perspectives.
Data were collected through PubMed using specified search criteria based on preselected topics. For clinical use, focus was put on model indications as surgical site infection, burns and chronic wounds e.g. diabetic foot ulcer. Articles from personal bibliography and the clinical experience of the authors were also taken into account, especially in areas of limited published information.
2. Importance of antibiotics and antiseptics in wound healing
An increasing emergence of resistance to topical and systemic antibiotics is observed [4], [6]. Antiseptics, as an alternative for topical wound treatment, tend to be microbicidal and have a broader spectrum of antimicrobial activity than antibiotics [6]. Furthermore, in comparison to most antibiotics, antiseptics reduce the likelihood of resistance emerging due to their multiple mechanisms of action targeting various aspects of cell biology in microbes [4]. Therefore, the use of topical antibiotics should be discouraged if appropriate antiseptics are available [7]. Furthermore, a recent WHO guideline advocates the use of good antisepsis peri-operatively while reducing the use of systemic antibiotics [8], [9].
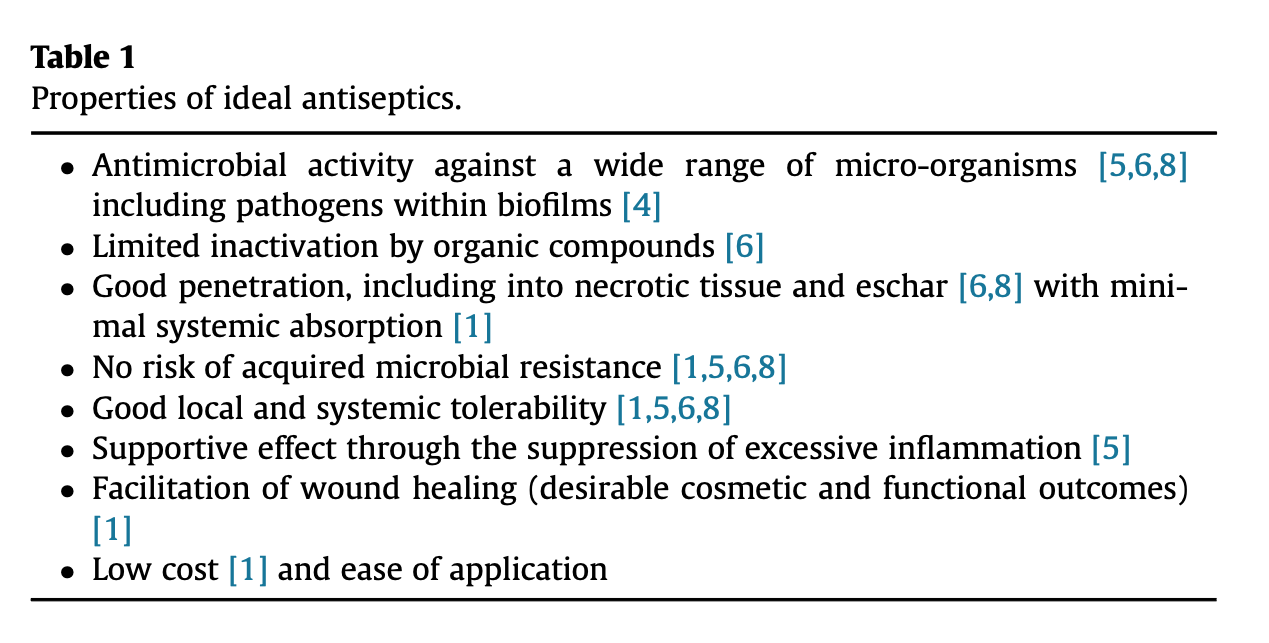
Overall, the properties of an ideal antiseptic include a broad spectrum of activity [5], [6], [10], the ability to penetrate biofilms [4], necrotic tissue, and eschar [4], [6], [10], a low potential for acquired resistance [1], [5], [6], [10], supportive effects for wound healing by impeding excessive inflammation [5], [11], and good local tolerability [5], [6], [10] (Table 1). Few antiseptics remain to be relevant for the prevention and treatment of infection in wound care. These include iodine carriers (iodophores) with polyvinylpyrrolidone (PVP or povidone) iodine (which will be discussed in detail as the most prominent representative), as well as silver, chlorhexidine, benzalkonium chloride, triclosan, octenidine, and polihexanide (PHMB) and selected dyes such as Eosine.
3. Povidone iodine
The role of iodine in wound care is primarily as an antimicrobial agent. Povidone iodine has been used and tested in wound healing for many decades [4], [10]. As with other antiseptics, in vitro, animal, and clinical data using various formulations and concentrations in studies of differing design, endpoints, and quality continuously accumulates, while some questions still remain to be answered [12], [13].
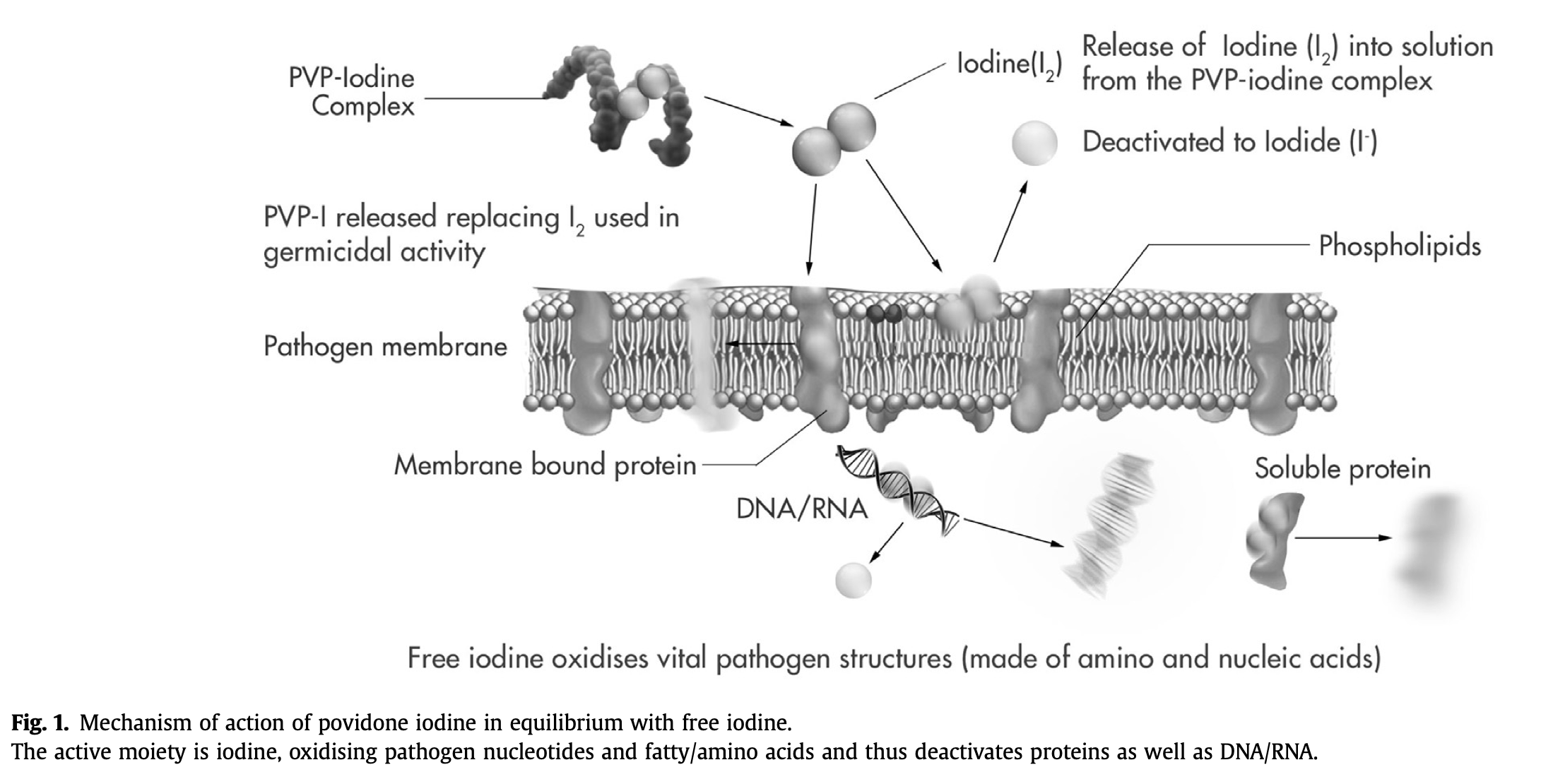
In povidone iodine, iodine forms a complex with the synthetic carrier polymer povidone, which itself has no microbicidal activity [10]. In an aqueous medium, free iodine is released into solution from the povidone iodine complex and an equilibrium is established, with more free iodine being released from the povidone iodine reservoir as iodine-consuming germicidal activity proceeds (Fig. 1) [14], [15].
Fig. 1. Mechanism of action of povidone iodine in equilibrium with free iodine.
The active moiety is iodine, oxidising pathogen nucleotides and fatty/amino acids and thus deactivates proteins as well as DNA/RNA.
The formulation-, concentration- and temperature-dependent equilibrium of povidone-bound iodine to free iodine serves to minimize safety and tolerability issues associated with skin exposure to earlier elemental iodine formulations [10], and appears to protect against inhibition of granulation tissue formation [16].
3.1. Mode of action
The microbicidal activity of iodine appears to involve the inhibition of vital bacterial cellular mechanisms and structures, and oxidizes nucleotides fatty/amino acids in bacterial cell membranes, in addition to cytosolic enzymes involved in the respiratory chain, causing them to become denatured and deactivated (Fig. 1) [17]. However, the precise sequence of events occurring at the molecular level has yet to be fully elucidated [6], [14], [15], [18], [19]. Cytotoxicity studies have shown that the bactericidal effect occurs even before individual human cells are affected (see below).
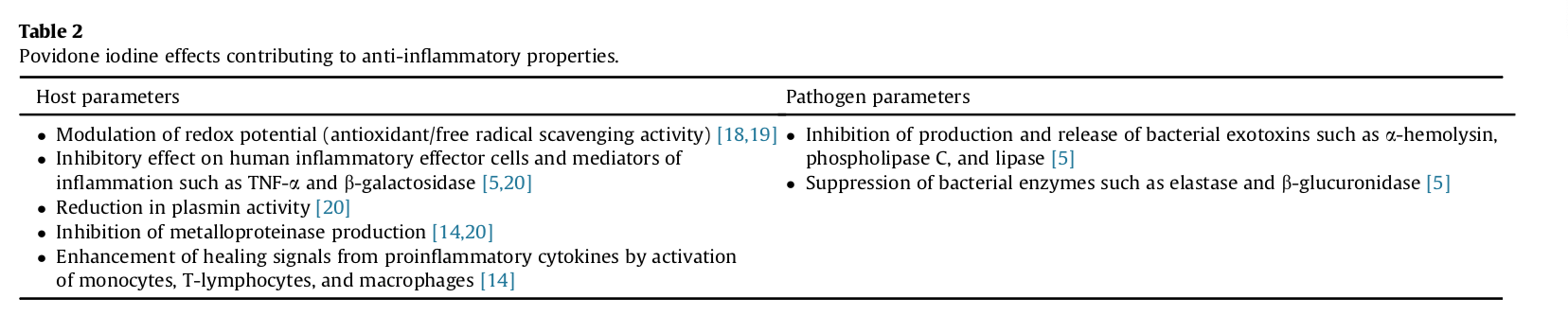
In vitro evidence suggests that iodine not only has broad spectrum antibacterial effects, but also counteracts inflammation elicited by both pathogens and the host response [20]. These anti-inflammatory effects appear to be multifactorial (Table 2) [5], [16], [21], [22] and have been shown to be clinically relevant [21], [23].
3.2. Preclinical efficacy data
3.2.1. Spectrum of activity
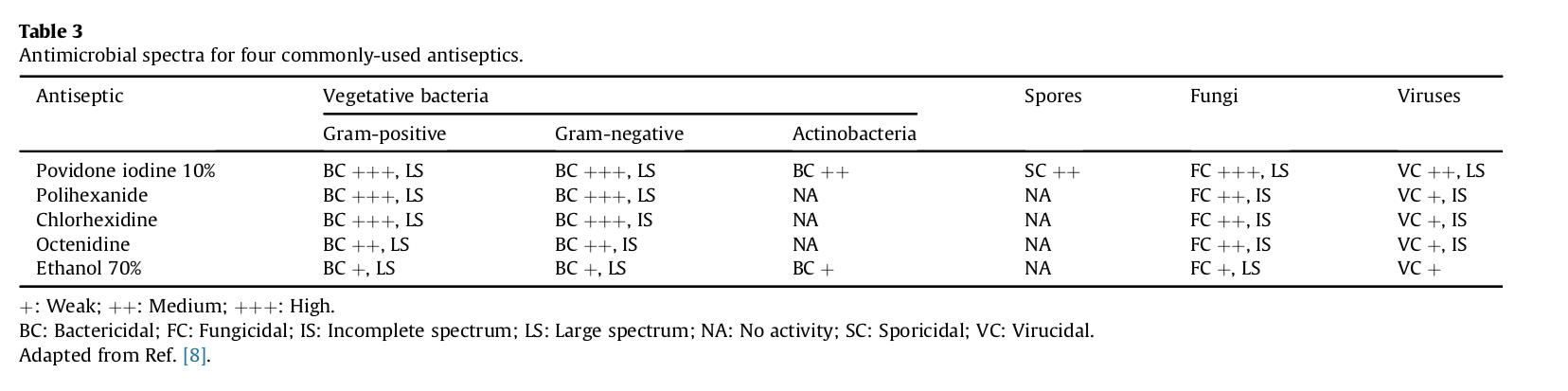
Povidone iodine is one of the few topical antimicrobial shown to be effective against bacteria, several viruses, fungi, spores, protozoa, and amoebic cysts (Table 3) [6], [10], [11], [14], [24].
+: Weak; ++: Medium; +++: High.
BC: Bactericidal; FC: Fungicidal; IS: Incomplete spectrum; LS: Large spectrum; NA: No activity; SC: Sporicidal; VC: Virucidal.
Adapted from Ref. [8].
In classical antimicrobial testing, povidone iodine has been shown to kill a variety of bacterial strains known to commonly cause nosocomial infections, including methicillin-resistant Staphylococcus aureus (MRSA) and other antibiotic-resistant strains within 20–30 s of exposure [25], [27]. In contrast, comparators such as chlorhexidine require much longer exposure times, and residual bacteria persist for most species [25], [27].
3.2.2. Resistance and cross-resistance
The global prevalence of hospital- and community-acquired infections is rapidly increasing, together with increasing resistance to topical antibiotics such as mupirocin, fusidic acid, and gentamicin, as well as systemic antibiotics [6], [28], [29], [30]. This poses a major medical challenge, and appears to be partially attributable to the overuse and misuse of antibiotics [31], [32].
Most data exists on bacterial resistance and cross-resistance to antiseptics, including chlorhexidine, quaternary ammonium salts, silver and triclosan, as reported in strains isolated from clinical settings [6], [16], [26], [27], [33]. Evidence of cross-resistance between antiseptics and antibiotics has also been documented [16], [34]. Furthermore, resistance to povidone iodine has not been induced in systematic testing to date [35]. Thus, in contrast to other antiseptics (with the apparent exception of an absence of cross-resistance to silver), no acquired resistance or cross-resistance has been reported for iodine in over 150 years of use [6], [14], [16], [26], [27]. This lack of resistance is likely due to iodine's multiple mechanisms of action [36].
3.2.3. Activity against biofilms
Biofilms are known to delay wound healing and can promote bacterial survival in the presence of antimicrobial therapies [37]. The sustained efficacy of povidone iodine on wound healing in the presence of biofilms has been recently reviewed [38]. Studies have confirmed the in vitro efficacy of povidone iodine against S. epidermidis and S. aureus growth, as well as the inhibition of staphylococcal biofilm formation at sub-inhibitory concentrations [39]. Furthermore, in a CDC-reactor model, povidone iodine was efficacious in the presence of biofilms grown in a mixed culture comprising MRSA and C. albicans, even at highly diluted concentrations [40]. The biofilm removal in this assay was greater than observed with e.g. PHMB, octenidine, chlorhexidine, mupirocin and fusidic acid [40].
3.2.4. Toxicity, tolerability, and allergenicity
A number of cytotoxicity studies have been conducted to investigate the potential detrimental effects of povidone iodine and other antiseptics on wound healing typically fibroblasts, keratinocytes and other cell lines. While all antiseptics may have a measure of cytotoxicity due to nonspecific effects, this may not necessarily be clinically relevant to the wound healing process [41]. Cytotoxicity data from tests performed on isolated cells must be considered in perspective. In vitro cytotoxicity can be more pronounced than in a biological system with a three-dimensional matrix and vascular system, and not necessarily reflective of in vivo or clinical settings [16], [41]. Interestingly, recent cytotoxicity tests have shown that povidone iodine has very low cytotoxicity compared to other antiseptics when tested on skin (vs. PHMB, octenidine, chlorhexidine and hydrogen peroxide) [41] and oromucosal (same as octenidine but superior to chlorhexidine) cell lines [42]. However, other studies have reached different conclusions. The inconsistency in translation may be further due to the multitude of protocols, cell types and species involved in the testing.
Clinical experience reveals increasing contact allergies to topical antibiotics such as neomycine [43] or fusidic acid [44], and antiseptics such as chlorhexidine [45], [46]. In addition, sensitisation through topical applications of antibiotics can induce severe generalized allergic reactions if these antibiotics are later used systemically and vice versa [47]. On the other hand proven allergies against povidone iodine or silver are rare [46]. Sensitisations against PVP-I are sometimes cross-reacting to iodinated contrast media [48]. However, these important cross-reactivities can be discovered by prick, intradermal and/or patch skin tests skin tests.
3.3. Non-clinical models
Animal models can sometimes lack clinical relevance, and appreciable differences exist between the dermal architecture in animal and human skin. Pig skin can compare closest to human skin and is therefore the most suitable non-human model. Rodent skin, in contrast, differs from humans considerably, as it is much thinner, the epidermis is synchronized to follicular cycles in the furry skin and it heals mainly by contraction rather than by migration of epidermal cells [49]. Animal models may further lack applicability to chronic wound care in clinical settings, as human patients often have underlying medical conditions that complicate healing [1]. These issues warrant caution when attempting to extrapolate preclinical data to clinical settings, and suggest that non-clinical data should be considered in conjunction with robust clinical evidence.
While some in vitro studies have suggested that povidone iodine may have a measure of cytotoxic effect [50], no consistent deleterious effects on various measures of wound healing have been demonstrated in in vivo studies, particularly at lower povidone iodine concentrations [41], [51]. A number of animal studies of povidone iodine in wound healing were published over thirty years ago [41], [52], and the majority demonstrated that concentrations of up to 10% generally do not inhibit the granulation and epithelialization processes [41].
Several animal studies have also investigated the effect of povidone iodine on wound microcirculation, with inconsistent findings. In rabbit ear chamber wounds, the use of a 5% povidone iodine solution was associated with an early but rapidly transient decrease in blood flow [51], [53]. However, in the 10% povidone iodine study mentioned earlier, wounds showed faster neovascularization with povidone iodine treatment compared with silver nitrate, sodium hypochlorite, and untreated controls [54]. Another rat model demonstrated no negative effects on capillary blood flow after up to 60 min exposure to a 1% povidone iodine solution [51].
Interestingly, a recent study has shown povidone iodine to enhance wound healing via TGF β, not only by increasing granulation but also enhancing neovascularization [55].
3.4. Data on povidone iodine safety
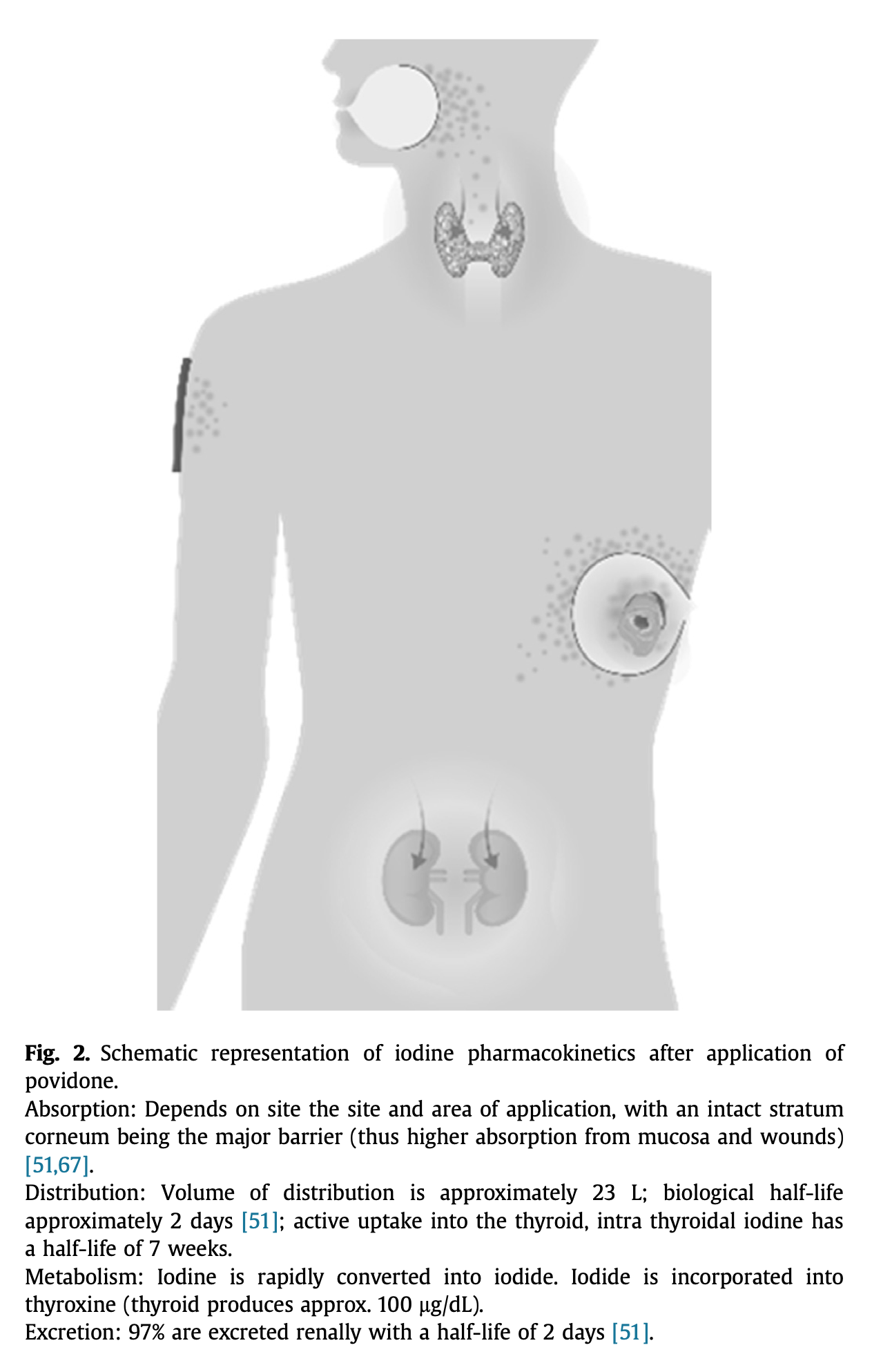
Data on the systemic absorption of antiseptics are scant [1]. Iodine seems to be absorbed from the skin, but more so from mucosa [56]. However, the condition of the skin barrier will determine transdermal iodine absorption. The absorption will be increased if the skin barrier is broken as in wounds and also dependent on skin age and surface area of application (Fig. 2). Safety information in povidone iodine product labeling includes general warnings against use in patients allergic to povidone iodine or excipients, thyroid disorders, in very low birth weight infants, and in patients receiving radio-iodine therapy [6], [57].
Fig. 2. Schematic representation of iodine pharmacokinetics after application of povidone.
Absorption: Depends on site the site and area of application, with an intact stratum corneum being the major barrier (thus higher absorption from mucosa and wounds) [51], [67].
Distribution: Volume of distribution is approximately 23 L; biological half-life approximately 2 days [51]; active uptake into the thyroid, intra thyroidal iodine has a half-life of 7 weeks.
Metabolism: Iodine is rapidly converted into iodide. Iodide is incorporated into thyroxine (thyroid produces approx. 100 μg/dL).
Excretion: 97% are excreted renally with a half-life of 2 days [51].
4. Clinical evidence of povidone iodine efficacy
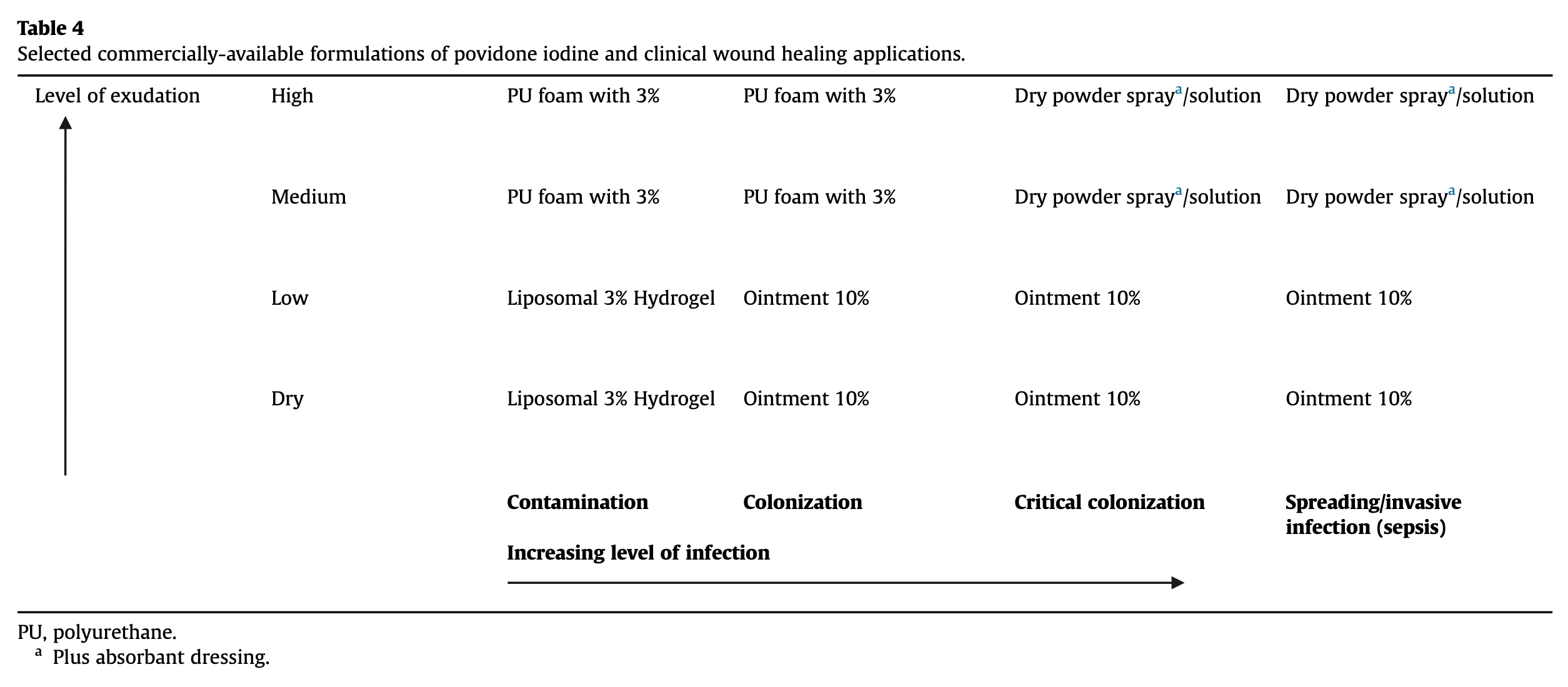
Human trials of povidone iodine in wound healing have varied widely in regards to inclusion criteria, demographic characteristics, underlying pathologies, medical interventions, and trial endpoints [10], [52]. Povidone iodine products have been available in many countries without prescription for decades [56], and are generally considered to be effective antiseptics that do not impede wound healing [12], [52]. A range of povidone iodine products have been formulated to suit specific medical needs, with current uses and benefits for various indications discussed below (summarized in Table 4) [10].
PU, polyurethane.
- a
-
Plus absorbant dressing.
The concentration of free iodine determines the germicidal action of povidone iodine [15], and the color of povidone iodine can be used as an indicator of maintenance of efficacy, and as a reminder to reapply the product [13]. In addition, color gradation can aid the practitioner in distinguishing between superficial and deep burns to guide wound debridement [58]. The frequency of dressing changes and type of povidone iodine formulation needed are dependent on the nature and behavior of the wound bed.
In therapeutic use, treatment should stop when symptoms subside. However, wound healing is more than antisepsis [2], [4] and a good choice of dressings and procedures is essential for the outcome. Therefore, povidone iodine antisepsis is one important measure amongst others. This includes good debridement, cleaning and choice of an appropriate dressing. Negative wound pressure therapy is getting increasingly popular, but also not a single solution as infection is a constant threat. Povidone iodine can be used both prophylactically during wound cleaning and therapeutically as leave-on application in contaminated chronic and acute wounds. A typical regimen for cleaning is a soaking time of 20 min each, using cycle frequencies of four to eight cycles per day [59].
4.1. Prevention of surgical site infection
Surgical site infections (SSI) continue to be a significant source of increased morbidity, mortality, hospital length of stay, and care costs [60]. As the majority of infections acquired during surgery originate from the patient's own commensal flora, disinfection of the surgical site prior to incision is imperative [61]. A large American study in 7669 clean-contaminated surgical patients [61], as well as a recent Cochrane review [60], confirmed the value of preoperative skin antisepsis with povidone iodine as a feasible clinical option. While there appeared to be little benefit from the addition of alcohol to povidone iodine [60], [61], one study of 200 healthy volunteers showed that the use of 70% isopropyl alcohol before or after 10% povidone iodine was more effective in reducing bacterial skin counts than disinfection with a single agent [62]. The recent WHO guideline prefers alcoholic chlorhexidine solution over povidone iodine for pre-surgical use [8], [9] However, some recent studies [63] were possibly not included due to a cut-off date leading to a recommendation in contrast to recent Cochrane Reviews.
As well as surgical site preparation, intraoperative flushing with povidone iodine has been shown to reduce infection rates in sensitive indications including breast surgery (at a 4% dilution) [64], spinal surgery (0.35% dilution) [65], total joint arthroplasty (0.35% dilution) [66], and intraperitoneal irrigation (1% dilution) during laparotomy [67]. Such wound irrigation with diluted povidone iodine has been advocated by a recent WHO guideline [8], [9]. In some countries, the use of povidone iodine dilutions represents approved usage except for pre-operative skin prepping, where full concentrations are needed to eliminate resident microflora.
4.2. Povidone iodine in acute wounds
Depending on severity, acute traumatic wounds may be subject to OTC treatment with clear guidance on when to invoke physician referral [68]. Such wounds may benefit from the use of povidone iodine, especially when contaminated or infected [69].
Dry powder sprays may be better tolerated in sensitive wounds while having a hemostaticeffect on some superficial bleeding, and can be applied without the need for wound dressing. However, ointments can increase the efficacy and absorption of Iodine by occlusion effects and can be used in dry wounds or skin and to prevent adhesion, but it is less indicated if oozing or exudate is predominant.
4.3. Surgical wounds
In a clinical trial of patients undergoing split skin grafts, the use of povidone iodine ointment medicated gauze did not delay wound healing when compared to simple vaseline gauze, and evidence for a possible earlier onset of epithelialization with povidone iodine was observed [23]. In addition, wounds treated with povidone iodine trended toward lower bacterial counts in comparison to the gauze controls.
In graft wounds, the efficacy of liposomal povidone iodine hydrogel has been demonstrated to significantly enhance antiseptic efficacy, wound epithelialization, healing quality, and graft take when compared with chlorhexidine gauze, with no clinically-relevant topical or systemic adverse effects reported [69], [70].
In contaminated and dirty wounds requiring surgical attention (class III and IV), antisepsis (together with systemic antibiosis and a check on the tetanus immunization status) is warranted. Intense debridement and wound cleaning, together with suitable wound treatment devices such as polyurethane foams may facilitate long term healing. Dressing changes and antisepsis can be delegated to family members, allowing for easier follow-up in countries with slim medical infrastructure. Dry powder sprays for exuding wounds and ointments for drier wounds allow differentiated treatment. Patients have reported the additional benefit of a cooling effect experienced from dry powder sprays, likely due to the evaporating propellant.
4.4. Burn wounds
The potential for microbial infection is high in burn wounds, and infection can cause partial thickness burns to progress to full thickness, with severe consequences [21]. Burns precipitate a mediator-induced response, with evidence of both local and systemic oxidative changes, increased oxygen free radical activity, lipid peroxidation, and a reduction in antioxidant scavenging capacity [21].
In a study of 38 patients with varying burn sizes, the addition of povidone iodine ointment to a standard hospital antibiotic protocol alone or in combination with daily systemic vitamins E and C improved markers of oxidative stress and wound healing. These effects were accompanied by a 15.3% decrease in the incidence of infection after 4 days of treatment compared with zero time, a reduction in the mortality rate from 87.5% for those receiving standard care to 5.9% in povidone iodine-treated patients, a faster wound healing time, and reduced hospital cost burden [21]. Furthermore, no adverse systemic effects were observed. The effect of povidone iodine on neutrophils [5] and oxygen radicals [20] is potentially of significant value in burns patients due to the limitations imposed by collateral tissue damage [71].
A 5% povidone iodine cream was reported to be superior to silver sulfadiazine in terms of application and wound healing [72]. This formulation was also shown to be effective and well tolerated in an uncontrolled pediatric trial [73].
In another randomized trial involving 213 patients with partial thickness burns, treatment with a povidone iodine dressing was associated with reduced treatment times, lower analgesiarequirements, fewer hospital visits, and less time off work compared with chlorhexidine dressing treatment [74]. Interestingly, a trend toward less pain and bleeding on dressing removal was also observed with povidone iodine [74]. Another trial in a similar patient population demonstrated faster wound healing and a more favorable cosmetic result with liposomal povidone iodine hydrogel versus silver sulfadiazine [75].
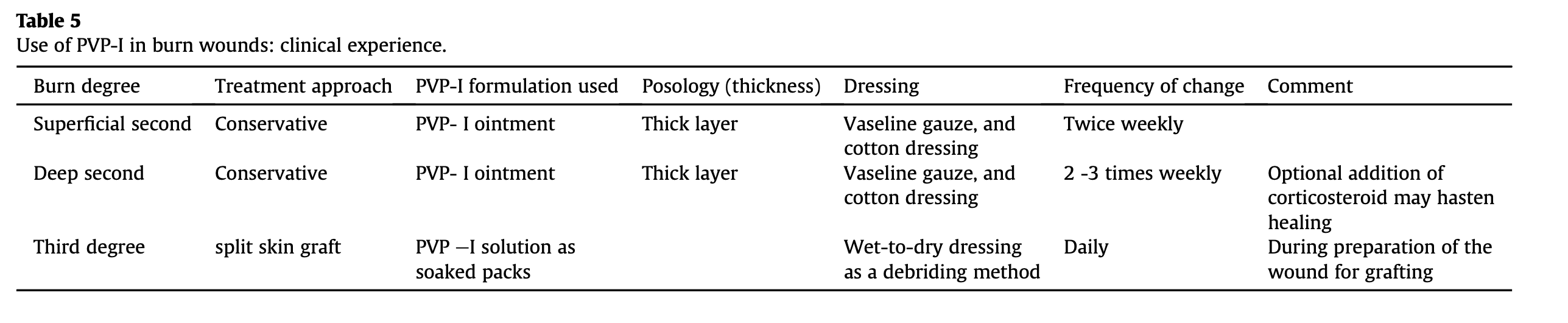
The authors' clinical experience with the use of povidone iodine in patients with burn wounds is detailed in Table 5.
4.5. Povidone iodine in chronic wounds
Chronic skin wounds affect 1–3% of the population [76] and represent a significant clinical and financial burden to healthcare systems [22], with approximately 50% of outpatients requiring nursing services [77]. In most cases, chronic underlying disease undermines normal tissue repair, preventing wounds from healing as expected even after intense treatment for an extended amount of time [2], [4], [22].
Chronic wounds often harbor biofilms that render the infection more difficult to detect, diagnose and treat [4], [32], [37]. A shift towards anaerobic and gram negative bacteriaseems to occur with increasing chronicity of wounds [3].A lack of perfusion in these chronic wounds with insufficiency of the arterial and/or venous system does not support the routine use of systemic antibiotics to promote the healing of chronic wounds. Consensus suggests that systemic antibiotics should only be used in cases of established clinical infection [7], [78]. In addition, about half patients with chronic wounds develop allergic contact eczemas to topical products applied, particularly to preservatives, emollients, dressing adhesives, antibiotics (e.g. fucidic acid or neomycine) and antiseptics (e.g. chlorhexidine). Therefore, the use of hypoallergenic formulations and active ingredients are important to avoid sensibilisations through the treatment of wounds. Povidone iodine and silver have in this aspect a very good safety profile.
The benefits of povidone iodine in chronic wound healing, as in burn wounds [23], may extend beyond its antimicrobial properties [74].
4.5.1. Venous and arterio-venous leg ulcers
In an intra-individual comparison study, 51 patients with at least two similar chronic leg ulcers were treated with hydrocolloid dressings. One ulcer in each patient received additional topical application of povidone iodine, silver sulfadiazine, or chlorhexidine. While comparable antimicrobial activity was observed with all three agents, povidone iodine exposure resulted in a highly significant 2–9 week improvement in healing time, with superior microvascularity and dendrocyte density [79]. In another study of female patients with at least two chronic venous leg ulcers treated with hydrocolloid dressings, daily application of povidone iodine solution resulted in a faster reduction in ulcer size, a lower bacterial load, and less pronounced inflammatory effects versus hydrocolloid dressing alone [80]. Long-term local application of povidone iodine solution and ointment in a study of 25 patients with venous leg ulcers (VLUs) secondary to chronic primary lymphedema also resulted in excellent local tolerability and clinical efficacy, with no evidence of resistance [81].
In a further study of 63 patients with VLUs, 42 had compression bandages applied, of whom 21 with superficial infection were treated locally with povidone iodine, and 21 received systemic antibiotics [76]. The remaining 21 patients were treated locally with povidone iodine without compression. Unsurprisingly, compression increased the ulcer healing rate; furthermore, healing rates among patients receiving systemic antibiotics were comparable to those treated with topical povidone iodine, but the use of systemic antibiotics increased the risk of relapse with superficial bacterial infections (impetigo and folliculitis) compared with local povidone iodine application (32% vs. 11%, respectively).
4.5.2. Diabetic foot ulcers
In a retrospective study of 30 patients with a total of 42 wounds, primarily diabetic foot ulcers, 29% of wounds achieved full closure and 45% achieved partial closure within 6 months with regular topical povidone iodine application [77]. Another study in ulcer patients (the majority being diabetic) revealed less pain associated with povidone iodine dressings compared with cadexomer-iodine and silver dressings [82]. Under some conditions, diabetic foot ulcers may be treated by split thickness skin grafting or allografts, and povidone iodine antisepsis can help ensure graft success by reducing the bacterial load in cost-sensitive environments [83]. However, the detailed effects of these antiseptics on skin grafts require further evaluation in standardized clinical trials.
4.5.3. Pressure ulcers
In a randomized study of 27 spinal cord injury patients with pressure ulcers, 84% of ulcers treated with povidone iodine hydrogel achieved re-epithelialization, versus 54% treated with povidone iodine solution and gauze; however, there was no significant difference in the speed of healing (measured in cm2/day) between the two groups. The authors concluded that the moist environment created by the hydrogel, rather than the use of a dry gauze dressing, was the most likely reason for the higher rate of epithelialization raising questions with respect to the experimental design [84]. In another study, 18 outpatients with pressure ulcers and stasis ulcers received daily application of povidone iodine ointment under and within the dressing, which resulted in a reduced level of infection and inflammation, and apparent promotion of healing [85].
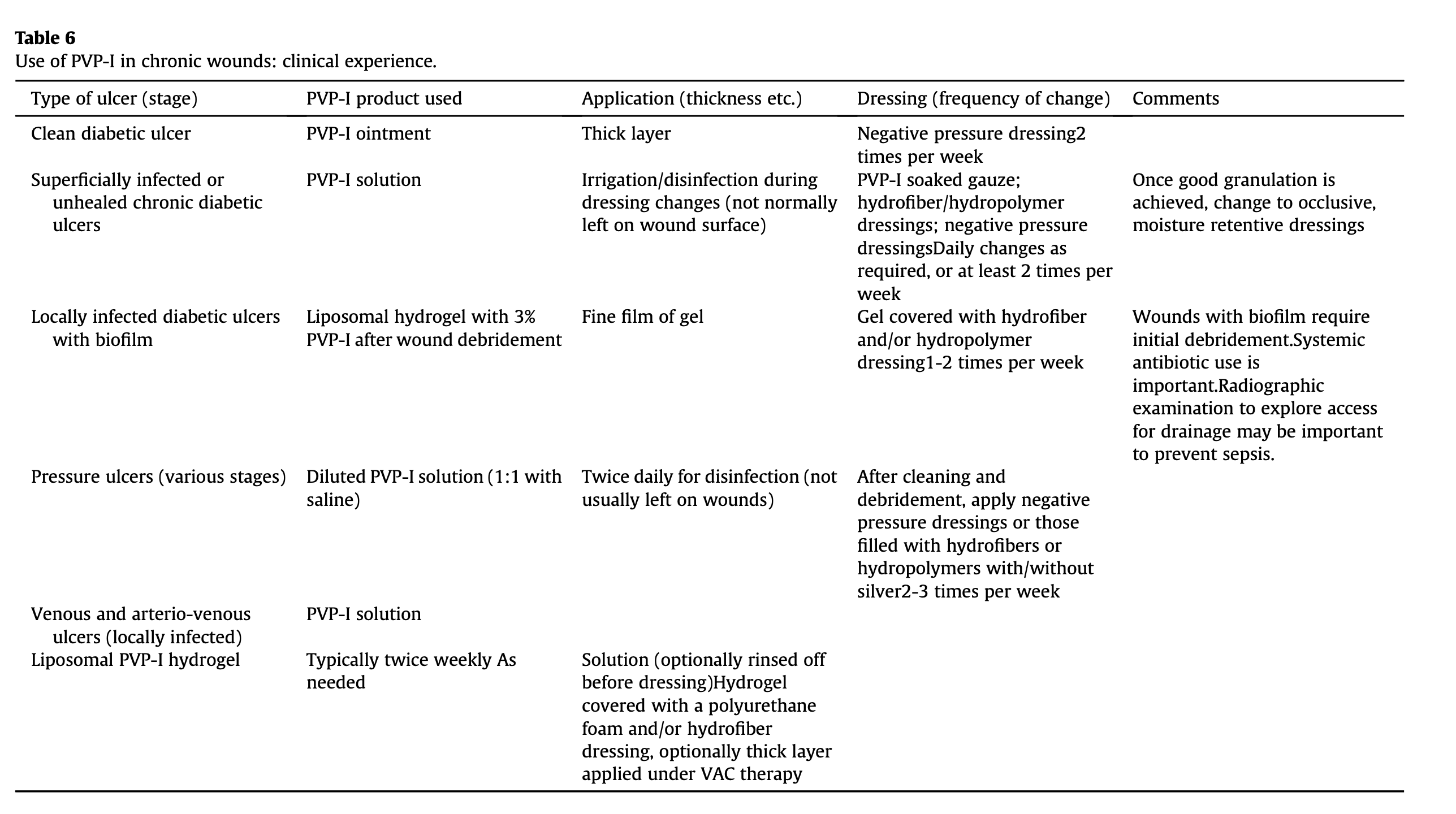
The authors' general clinical experiences with povidone iodine use in the treatment of chronic wounds have been summarized in Table 6. It is acknowledged that an individualized approach should be taken, depending upon specific wound and patient characteristics.
5. Summary
Mounting evidence suggests that antiseptics should be used in preference to topical and systemic antibiotics for localized skin infections. Povidone iodine, the most commonly used iodophor, has many characteristics that position it extraordinarily well for wound healing, including its broad antimicrobial spectrum, lack of resistance, efficacy against biofilms, good tolerability and its effect on excessive inflammation. Clinical alternatives include chlorhexidine, silver-containing products, PHMB, octenidine and the old antiseptic dyes, although they all have different overall profiles that must be taken into consideration when making a chosing an antiseptic. Of clinical relevance, wound healing in clean wounds is not impeded and in colonised wounds is supported [22], [67]. Its use is especially important for sensitive and difficult to treat wounds and in the case of chronic wounds where an extended duration of therapy is likely. Some combinations as medicated gauzes make known formulations easier to apply. Newer application methods and formulations, such as inclusion in wound healing devices or carrier systems such as liposomes have extended the reach of existing formulations by combining iodine with modern wound treatment concepts. Due to its rapid, potent, broad-spectrum antimicrobial properties, and favorable risk/benefit profile, povidone iodine is expected to remain a highly effective treatment for acute and chronic wounds in the foreseeable future.
Ethical approval
No ethical approval for this review.
Funding
This review was received public funding through A*STAR and NUH Singapore, and was supported by an educational grant from Mundipharma Pte Ltd, Singapore.
Author contribution
The Authors: Paul Lorenz Bigliardi, Syed Abdul Latiff Alsagoff, Hossam Yehia El-Kafrawi, Jai-Kyong Pyon, Chad Tse Cheuk Wa and Martin Anthony Villa were all involved with (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article and revising it, and (3) final approval of the version to be submitted.
Conflicts of interest
No Conflicts.
Paul Lorenz Bigliardi confirms that I have given academic presentations for Mundipharma on wound healing and was consulting them.
Research registration unique identifying number (UIN)
No research registration unique identifying number for this review.
Guarantor
Professor Paul Lorenz Bigliardi.
Acknowledgements
The authors thank Lee Farrand for providing medical editorial support, which was funded by Mundipharma Pte Ltd, Singapore.
References
- [1]
-
B.A. Lipsky, C. HoeyTopical antimicrobial therapy for treating chronic woundsClin. Infect. Dis., 49 (2009), pp. 1541-1549
- [2]
-
G.S. Schultz, R.G. Sibbald, V. Falanga, E.A. Ayello, C. Dowsett, K. Harding, M. Romanelli, M.C. Stacey, L. Teot, W. VanscheidtWound bed preparation: a systematic approach to wound managementWound Repair Regen., 11 (Suppl 1) (2003), pp. S1-S28
- [3]
-
R. Edwards, K.G. HardingBacteria and wound healingCurr. Opin. Infect. Dis., 17 (2004), pp. 91-96
- [4]
-
D.J. Leaper, G. Schultz, K. Carville, J. Fletcher, T. Swanson, R. DrakeExtending the TIME concept: what have we learned in the past 10 yearsInt. Wound J., 9 (Suppl 2) (2012), pp. 1-19
- [5]
-
B. Konig, K. Reimer, W. Fleischer, W. KonigEffects of Betaisodona on parameters of host defenseDermatology, 195 (Suppl 2) (1997), pp. 42-48
- [6]
-
J.-M. Lachapelle, O. Castel, A. Fueyo CasadoAntiseptics era Bact. Resist. a focus povidone iodine Future Med, 10 (2013), pp. 579-592
- [7]
-
European Wound Management Association (EWMA)Position Document: Management of Wound InfectionLondon(2006)
- [8]
-
WHOGlobal Guidelines for the Prevention of Surgical Site InfectionISBN 978 92 4 154988 2World Health Organization (2016)2016
- [9]
-
B. Allegranzi, B. Zayed, P. Bischoff, N.Z. Kubilay, S. de Jonge, F. de Vries, S.M. Gomes, S. Gans, E.D.Wallert, X. Wu, M. Abbas, M.A. Boermeester, E.P. Dellinger, M. Egger, P. Gastmeier, X. Guirao, J. Ren, D. Pittet, J.S. SolomkinWHO Guidelines Development Group, New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspectiveLancet Infect. Dis., 16 (2016), pp. e288-e303
- [10]
-
S. Ripa, R. Bruno, R. RederClinical applications of Povidone-Iodine as a topical antimicrobialHandbook of Topical Antimicrobials Industrial Applications, Industrial applications in consumer products and Pharmaceuticals: CRC Press (2002)
- [11]
-
K. Jayaraja Kumar, E. Jayachandran, C. Hemanth Kumar ReddyApplication of broad spectrum antiseptic povidone iodine as powerful action: a reviewJ Pharm Sci. Technol, 1 (2009), pp. 48-58
- [12]
-
H. BanwellWhat is the evidence for tissue regeneration impairment when using a formulation of PVP-I antiseptic on open wounds?Dermatology, 212 (Suppl 1) (2006), pp. 66-76
- [13]
-
G. Selvaggi, S. Monstrey, K. Van Landuyt, M. Hamdi, P. BlondeelThe role of iodine in antisepsis and wound management: a reappraisalActa Chir. Belg, 103 (2003), pp. 241-247
- [14]
-
W. Fleischer, K. ReimerPovidone-iodine in antisepsis–state of the artDermatology, 195 (Suppl 2) (1997), pp. 3-9
- [15]
-
H. RackurNew aspects of mechanism of action of povidone-iodineJ. Hosp. Infect., 6 (Suppl A) (1985), pp. 13-23
- [16]
-
D.J. Leaper, P. DuraniTopical antimicrobial therapy of chronic wounds healing by secondary intention using iodine productsInt. Wound J., 5 (2008), pp. 361-368
- [17]
-
J. Kanagalingam, R. Feliciano, J.H. Hah, H. Labib, T.A. Le, J.C. LinPractical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infectionsInt. J. Clin. Pract., 69 (2015), pp. 1247-1256
- [18]
-
A.J. Mangram, T.C. Horan, M.L. Pearson, L.C. Silver, W.R. JarvisGuideline for prevention of surgical site infection, 1999. Centers for disease control and prevention (CDC) hospital infection control practices advisory committeeAm. J. Infect. Control, 27 (1999), pp. 97-132quiz 133-134; discussion 196
- [19]
-
H. Schreier, G. Erdos, K. Reimer, B. Konig, W. Konig, W. FleischerMolecular effects of povidone-iodine on relevant microorganisms: an electron-microscopic and biochemical studyDermatology, 195 (Suppl 2) (1997), pp. 111-116
- [20]
-
C.J. Beukelman, A.J. van den Berg, M.J. Hoekstra, R. Uhl, K. Reimer, S. MuellerAnti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel) for wound healing in vitro, Burns34 (2008), pp. 845-855
- [21]
-
A.A. Al-Kaisy, A. Salih SahibRole of the antioxidant effect of vitamin e with vitamin C and topical povidone-iodine ointment in the treatment of burnsAnn. Burns Fire Disasters, 18 (2005), pp. 19-30
- [22]
-
S.A. Eming, S. Smola-Hess, P. Kurschat, D. Hirche, T. Krieg, H. SmolaA novel property of povidon-iodine: inhibition of excessive protease levels in chronic non-healing woundsJ. Invest. Dermatol, 126 (2006), pp. 2731-2733
- [23]
-
M. Vehmeyer-Heeman, E. Van den Kerckhove, K. Gorissen, W. BoeckxPovidone-iodine ointment: no effect of split skin graft healing time, Burns31 (2005), pp. 489-494
- [24]
-
R.A. CooperIodine revisitedInt. Wound J., 4 (2007), pp. 124-137
- [25]
-
T. Yasuda, Y. Yoshimura, H. Takada, S. Kawaguchi, M. Ito, F. Yamazaki, J. Iriyama, S. Ishigo, Y. AsanoComparison of bactericidal effects of commonly used antiseptics against pathogens causing nosocomial infections. Part 2Dermatology, 195 (Suppl 2) (1997), pp. 19-28
- [26]
-
T. Yasuda, S. Yoshimura, Y. Katsuno, H. Takada, M. Ito, M. Takahashi, F. Yahazaki, J. Iriyama, S. Ishigo, Y. AsanoComparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureusPostgrad. Med. J., 69 (Suppl 3) (1993), pp. S66-S69
- [27]
-
T. Kunisada, K. Yamada, S. Oda, O. HaraInvestigation on the efficacy of povidone-iodine against antiseptic-resistant speciesDermatology, 195 (Suppl 2) (1997), pp. 14-18
- [28]
-
R.H. Demling, B. WaterhouseThe increasing problem of wound bacterial burden and infection in acute and chronic soft-tissue wounds caused by methicillin-resistant Staphylococcus aureusJ. Burns Wounds, 7 (2007), p. e8
- [29]
-
M.J. Ellington, S. Reuter, S.R. Harris, M.T. Holden, E.J. Cartwright, D. Greaves, S.M. Gerver, R. Hope, N.M. Brown, M.E. Torok, J. Parkhill, C.U. Koser, S.J. PeacockEmergent and evolving antimicrobial resistance cassettes in community-associated fusidic acid and meticillin-resistant Staphylococcus aureusInt. J. Antimicrob. Agents, 45 (2015), pp. 477-484
- [30]
-
D.J. Hetem, M.J. BontenClinical relevance of mupirocin resistance in Staphylococcus aureusJ. Hosp. Infect., 85 (2013), pp. 249-256
- [31]
-
T.M. Barbosa, S.B. LevyThe impact of antibiotic use on resistance development and persistenceDrug Resist Updat, 3 (2000), pp. 303-311
- [32]
-
R.S. Howell-Jones, M.J. Wilson, K.E. Hill, A.J. Howard, P.E. Price, D.W. ThomasA review of the microbiology, antibiotic usage and resistance in chronic skin woundsJ. Antimicrob. Chemother., 55 (2005), pp. 143-149
- [33]
-
S. Lanjri, J. Uwingabiye, M. Frikh, L. Abdellatifi, J. Kasouati, A. Maleb, A. Bait, A. Lemnouer, M.ElouennassIn vitro evaluation of the susceptibility of Acinetobacter baumannii isolates to antiseptics and disinfectants: comparison between clinical and environmental isolatesApr 11Antimicrob. Resist Infect. Control, 6 (2017), p. 36, 10.1186/s13756-017-0195-yeCollection 2017
- [34]
-
R. Chuanchuen, K. Beinlich, T.T. Hoang, A. Becher, R.R. Karkhoff-Schweizer, H.P. SchweizerCross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJAntimicrob. Agents Chemother., 45 (2001), pp. 428-432
- [35]
-
E.T. Houang, O.J. Gilmore, C. Reid, E.J. ShawAbsence of bacterial resistance to povidone iodineJ. Clin. Pathol., 29 (1976), pp. 752-755
- [36]
-
R.G. Sibbald, D. Leaper, D. QueenIodine made easyWounds Int., 2 (2011), p. 2
- [37]
-
P. Phillips, R. Wolcott, J. Fletcher, G. SchultzBiofilms made easyWounds Int., 1 (2010), pp. 1-6
- [38]
-
S.L. Percival, S. Finnegan, G. Donelli, C. Vuotto, S. Rimmer, B.A. LipskyAntiseptics for treating infected wounds: efficacy on biofilms and effect of pHCrit. Rev. Microbiol. (2014), pp. 1-17
- [39]
-
K.O. Oduwole, A.A. Glynn, D.C. Molony, D. Murray, S. Rowe, L.M. Holland, D.J. McCormack, J.P. O'GaraAnti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureusJ. Orthop. Res., 28 (2010), pp. 1252-1256
- [40]
-
M.J. Hoekstra, S.J. Westgate, S. MuellerPovidone-iodine ointment demonstrates in vitro efficacy against biofilm formationInt. Wound J., 14 (2017)172–17
- [41]
-
S.J. van Meurs, D. Gawlitta, K.A. Heemstra, R.W. Poolman, H.C. Vogely, M.C. KruytSelection of an optimal antiseptic solution for intraoperative irrigation: an in vitro studyJ. Bone Jt. Surg. Am., 96 (2014), pp. 285-291
- [42]
-
J. Schmidt, V. Zyba, K. Jung, S. Rinke, R. Haak, R.F. Mausberg, D. ZiebolzCytotoxic effects of octenidine mouth rinse on human fibroblasts and epithelial cells - an in vitro studyDrug Chem. Toxicol. (2015), pp. 1-9
- [43]
-
V.M. Sheth, S. WeitzulPostoperative topical antimicrobial useDermatitis, 19 (2008), pp. 181-189
- [44]
-
M.R. Park, D.S. Kim, J. Kim, K. AhnAnaphylaxis to topically applied sodium fusidateAllergy Asthma Immunol. Res., 5 (2013), pp. 110-112
- [45]
-
M. Bubenhofer, M. Fricker, U. Weber-Mani, A. HelblingChlorhexidine: a retrospective observational study of a potentially life-threatening moleculeJ. Investig. Allergol. Clin. Immunol., 25 (2015), pp. 152-154
- [46]
-
J.M. LachapelleA comparison of the irritant and allergenic properties of antisepticsEur. J. Dermatol, 24 (2014), pp. 3-9
- [47]
-
R. Mariappan, P. Manninen, E.M. Massicotte, A. BhatiaCirculatory collapse after topical application of vancomycin powder during spine surgeryJ. Neurosurg. Spine, 19 (2013), pp. 381-383
- [48]
-
S. Carvalho, J. Marcelino, F. Cabral Duarte, A.C. Costa, M. Pereira BarbosaIs ‘Iodine Allergy’ a contraindication to iodinated contrast media? The spread of a mythAllergy, 71 (Suppl. 102) (2016), pp. 413-504
- [49]
-
E. Middelkoop, A.J. van den Bogaerdt, E.N. Lamme, M.J. Hoekstra, K. Brandsma, M.M. UlrichPorcine wound models for skin substitution and burn treatmentBiomaterials, 25 (2004), pp. 1559-1567
- [50]
-
A.K. Balin, L. PrattDilute povidone-iodine solutions inhibit human skin fibroblast growthDermatol Surg., 28 (2002), pp. 210-214
- [51]
-
R.I. BurksPovidone-iodine solution in wound treatmentPhys. Ther., 78 (1998), pp. 212-218
- [52]
-
H. Vermeulen, S.J. Westerbos, D.T. UbbinkBenefit and harm of iodine in wound care: a systematic reviewJ. Hosp. Infect., 76 (2010), pp. 191-199
- [53]
-
S.S. Brennan, D.J. LeaperThe effect of antiseptics on the healing wound: a study using the rabbit ear chamberBr. J. Surg., 72 (1985), pp. 780-782
- [54]
-
D. Kjolseth, J.M. Frank, J.H. Barker, G.L. Anderson, A.I. Rosenthal, R.D. Acland, D. Schuschke, F.R.Campbell, G.R. Tobin, L.J. WeinerComparison of the effects of commonly used wound agents on epithelialization and neovascularizationJ. Am. Coll. Surg., 179 (1994), pp. 305-312
- [55]
-
L. Wang, W. Qin, Y. Zhou, B. Chen, X. Zhao, H. Zhao, E. Mi, Q. Wang, J. NingTransforming growth factor β plays an important role in enhancing wound healing by topical application of Povidone-iodineSci. Rep., 9 (2017), p. 991
- [56]
-
B. Globel, H. Globel, C. AndresIodine resorption from PVP-iodine preparations after their use in humansDtsch. Med. Wochenschr, 109 (1984), pp. 1401-1404
- [57]
-
MIMS OnlineBetadine spray. General warnings for povidone-iodine[10 June, 2015]; Available from
- [58]
-
Y.S. Lau, P. BrooksInnovative use of povidone-iodine to guide burn wound debridement and predict the success of biobrane as a definitive treatment for burnsAdv. Skin. Wound Care, 27 (2014), pp. 111-113
- [59]
-
D.A. Back, C. Scheuermann-Poley, C. WillyRecommendations on negative pressure wound therapy with instillation and antimicrobial solutions - when, where and how to use: what does the evidence show?Int. Wound J., 10 (Suppl. 1) (2013), pp. 32-42
- [60]
-
J.C. Dumville, E. McFarlane, P. Edwards, A. Lipp, A. HolmesPreoperative skin antiseptics for preventing surgical wound infections after clean surgeryCochrane Database Syst. Rev. (2015), p. CD003949
- [61]
-
T.W. Hakkarainen, E.P. Dellinger, H.L. Evans, F. Farjah, E. Farrokhi, S.R. Steele, R. Thirlby, D.R. Flum, C. SurgicalOutcomes assessment program, comparative effectiveness of skin antiseptic agents in reducing surgical site infections: a report from the Washington state surgical care and outcomes assessment programJ. Am. Coll. Surg., 218 (2014), pp. 336-344
- [62]
-
S.S. Kim, S.B. Yu, J.D. Kim, S.J. RyuComparison of disinfective power according to application order of 70% isopropyl alcohol and 10% povidone-iodineKorean J. Anesthesiol., 65 (2013), pp. 519-524
- [63]
-
M. MaiwaldSkin preparation for prevention of surgical site infection after Cesarean delivery: a randomized controlled trialObstet. Gynecol. (2017), pp. 750-751
- [64]
-
S. Giordano, H. Peltoniemi, P. Lilius, A. SalmiPovidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative studyAesthet. Surg. J., 33 (2013), pp. 675-680
- [65]
-
M.T. Cheng, M.C. Chang, S.T. Wang, W.K. Yu, C.L. Liu, T.H. ChenEfficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgeryPhila Pa 1976Spine, 30 (2005), pp. 1689-1693
- [66]
-
N.M. Brown, C.A. Cipriano, M. Moric, S.M. Sporer, C.J. Della ValleDilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infectionJ. Arthroplasty, 27 (2012), pp. 27-30
- [67]
-
W.F. Sindelar, G.R. MasonIntraperitoneal irrigation with povidone-iodine solution for the prevention of intra-abdominal abscesses in the bacterially contaminated abdomenSurg. Gynecol. Obstet., 148 (1979), pp. 409-411
- [68]
-
W.S. PrayCaring for minor woundsU. S. Pharm., 31 (2006), pp. 16-23[62] M.N. Khan, A.H. Naqvi, Antiseptics, iodine, povidone iodine and traumatic wound cleansing, J Tissue Viability 16(2006) 6–10
- [69]
-
P.M. Vogt, J. Hauser, O. Rossbach, B. Bosse, W. Fleischer, H.U. Steinau, K. ReimerPolyvinyl pyrrolidone-iodine liposome hydrogel improves epithelialization by combining moisture and antisepis. A new concept in wound therapyWound Repair Regen., 9 (2001), pp. 116-122
- [70]
-
P.M. Vogt, K. Reimer, J. Hauser, O. Rossbach, H.U. Steinau, B. Bosse, S. Muller, T. Schmidt, W.FleischerPVP-iodine in hydrosomes and hydrogel–a novel concept in wound therapy leads to enhanced epithelialization and reduced loss of skin grafts, Burns32 (2006), pp. 698-705
- [71]
-
Y. Chen, W.G. JungerMeasurement of oxidative burst in neutrophilsMethods Mol. Biol., 844 (2012), pp. 115-124
- [72]
-
M. de Kock, A.E. van der Merwe, C. SwartsA comparative study of povidone iodine cream and silver sulphadiazine in the topical treatment of burnsS. Selwyn (Ed.), Proceedings of the First Asian/Pacific Congress on Antisepsis, Royal Society of Medicine Services Ltd (1988)
- [73]
-
P.M. de Wet, H. Rode, P. Matley, R. BrownA clinical assessment of the pharmacodynamics of 5% povidone iodine cream in burned childrenDermatology, 195 (1997), p. 155
- [74]
-
K.H. Han, A.K. MaitraManagement of partial skin thickness burn wounds with Inadine dressings, Burns15 (1989), pp. 399-402
- [75]
-
H.H. Homann, O. Rosbach, W. Moll, P.M. Vogt, G. Germann, M. Hopp, B. Langer-Brauburger, K. Reimer, H.U. SteinauA liposome hydrogel with polyvinyl-pyrrolidone iodine in the local treatment of partial-thickness burn woundsAnn. Plast. Surg., 59 (2007), pp. 423-427
- [76]
-
J. DaroczyQuality control in chronic wound management: the role of local povidone-iodine (Betadine) therapyDermatology, 212 (Suppl 1) (2006), pp. 82-87
- [77]
-
K.Y. WooManagement of non-healable or maintenance wounds with topical povidone iodineInt. Wound J., 11 (2014), pp. 622-626
- [78]
-
S. O'Meara, D. Al-Kurdi, Y. Ologun, L.G. Ovington, M. Martyn-St James, R. RichardsonAntibiotics and antiseptics for venous leg ulcersCochrane Database Syst. Rev. (2014), p. CD003557
- [79]
-
I. Fumal, C. Braham, P. Paquet, C. Pierard-Franchimont, G.E. PierardThe beneficial toxicity paradox of antimicrobials in leg ulcer healing impaired by a polymicrobial flora: a proof-of-concept studyDermatology, 204 (Suppl 1) (2002), pp. 70-74
- [80]
-
C. Pierard-Franchimont, P. Paquet, J.E. Arrese, G.E. PierardHealing rate and bacterial necrotizing vasculitis in venous leg ulcersDermatology, 194 (1997), pp. 383-387
- [81]
-
J. DaroczyAntiseptic efficacy of local disinfecting povidone-iodine (Betadine) therapy in chronic wounds of lymphedematous patientsDermatology, 204 (Suppl 1) (2002), pp. 75-78
- [82]
-
N. Campbell, D. CampbellEvaluation of a non-adherent, povidone-iodine dressing in a case series of chronic woundsJ. Wound Care, 22 (2013), pp. 401-402404-406
- [83]
-
S.M. Mahmoud, A.A. Mohamed, S.E. Mahdi, M.E. AhmedSplit-skin graft in the management of diabetic foot ulcersJ. Wound Care, 17 (2008), pp. 303-306
- [84]
-
A.Z. Kaya, N. Turani, M. AkyuzThe effectiveness of a hydrogel dressing compared with standard management of pressure ulcersJ. Wound Care, 14 (2005), pp. 42-44
- [85]
-
B.Y. Lee, F.S. Trainor, W.R. ThodenTopical application of povidone-iodine in the management of decubitus and stasis ulcersJ. Am. Geriatr. Soc., 27 (1979), pp. 302-306






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 