The Clinical Journal of Pain
Issue: Volume 32(11), November 2016, p 961–971
DOI: 10.1097/AJP.0000000000000352
|
Objectives: Osteoarthritis (OA) of the knee is one of the most common chronic diseases among older adults. This study aimed to test the effects of cabbage leaf wraps (CLWs) in the treatment of symptomatic OA.
|
|
Methods: Patients with OA of the knee at stages II to III (Kellgren-Lawrence) were randomly assigned to 4 weeks of treatment with CLWs (daily for at least 2h), topical pain gel (TPG) (10 mg diclofenac/g, at least once daily), or usual care (UC). The primary outcome measure was pain intensity (VAS) after 4 weeks. Secondary outcomes included functional disability Western Ontario and McMaster Universities Arthritis Index (WOMAC), quality of life (SF-36), self-efficacy (Arthritis Self-Efficacy Scale-D), physical function (30 s Chair Stand Test), pressure pain sensitivity (PPT), satisfaction, and safety after 4 and 12 weeks.
|
|
Results: In total, 81 patients were included in this study (42 women, 65.9±10.3 y). After 4 weeks patients in the CLW group reported significantly less pain compared with those in the UC group (difference, -12.1; 95% [confidence interval] CI, -23.1, -1.0; P=0.033) but not when compared with the TPG group (difference, -8.6; 95% CI, -21.5, 4.4; P=0.190). Significant effects were also found in WOMAC, SF-36, 30-second Chair Stand Test, and PPT scores in the CLW group compared with the UC group. Compared with TPG, effects from CLW were found for WOMAC after 4 weeks and for quality of life after 12 weeks. Patients were satisfied with both active interventions, and except for 2 adverse events in both groups the applications were well tolerated.
|
|
Conclusions: CLWs are more effective for knee OA than UC, but not compared with diclofenac gel. Therefore, they might be recommended for patients with OA of the knee. Further research is warranted.
|
|
Osteoarthritis (OA) of the knee is among the most common chronic diseases affecting the elderly, largely influencing their daily life activities.1 About one fourth of people over 55 years of age reported a significant knee pain episode in the last year.2 OA of the knee is characterized by articular cartilage destruction in addition to underlying bony changes at the joint margins.3 Main symptoms include pain and functional impairment, severely affecting patients’ quality of life.4
|
|
Conservative symptomatic treatment mainly consists of physiotherapy and pharmacological therapy.5,6 Self-care guides often recommend the use of wraps and compresses 7–9; however, high-quality trials are urgently needed before any conclusions can be drawn on such treatments.5
|
|
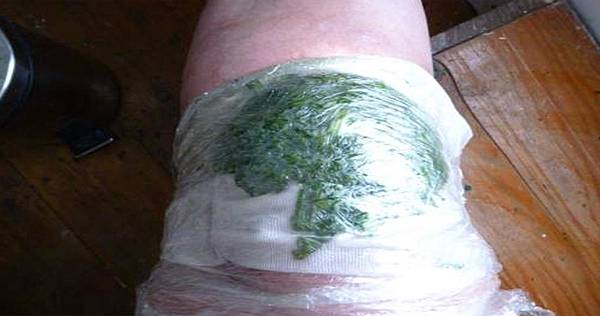
One such treatment that has been used for centuries is the cabbage leaf wrap (CLW).10 Cabbage leaves, preferably from white or savoy cabbage, are applied to the painful knee joint for several hours for pain relief. The health-promoting qualities of cabbage have been known for centuries,10 and they are being used for a variety of symptoms, such as breast engorgement.11,12 However, no studies have yet investigated the clinical potential of CLWs in patients with symptomatic knee OA.
|
|
This study aimed to investigate the effects of CLWs compared with usual care (UC) and a topical pain gel (TPG) to improve symptoms and the quality of life in patients with knee OA.
|
|
Ethical Approval and Trial Registration
|
|
The trial was conducted between September 2013 and July 2014 in the Department of Complementary and Integrative Medicine in Essen, Germany. The study was approved by the ethics committee of the University Hospital Essen (approval number: 13-5581-BO) and was registered at ClinicalTrials.gov (registry number: NCT02027792) before patient recruitment.
|
|
This was a randomized controlled 3-armed parallel-group trial. The intervention group was taught to apply CLWs themselves, whereas the control groups received either UC or a TPG (see the Interventions section). Written instructions were given to patients to explain the applications in detail. Each group received the allocated intervention for 4 weeks. measurements were taken at weeks 0, 4, and 12. At the trial’s end each patient received a financial reimbursement of 20 Euros.
|
|
Patients were recruited through local newspaper advertisements, with a medical student screening interested people by phone to assess their eligibility. Patients who met the inclusion criteria were invited for assessment, where they were provided with detailed written information about the study and their written informed consent was obtained. The study physician checked the patients’ medical histories, examined their physical health, and checked their medical records, such as laboratory findings, x-rays, or magnetic resonance imaging results. If patients met the inclusion criteria, and did not meet any exclusion criteria, they were included in the trial.
|
|
Trial participants were required to be at least 18 years of age and to have symptomatic knee OA at stages 2 or 3 according to the Kellgren-Lawrence classification 13 confirmed by medical records. They also had to report at least moderate pain of 45 mm or higher on a 0 to 100 mm visual analog scale (VAS),14 with 100 mm described as “worst knee pain imaginable.”
|
|
The trial exclusion criteria included pain due to secondary arthritis (eg, after an injury, inflammatory rheumatic diseases), prior injections with cortisone (within the past 4 wk) or hyaluronic acid (within the past 6 mo), prior operations to the knee (within the past 12 mo), or any severe comorbidity, such as liver or kidney diseases, asthma, or psychiatric disorders. Finally, pregnant or lactating women, patients who had recently started using corticosteroids or immunosuppressive drugs or other wraps and poultices for the knee were excluded.
|
|
Randomization and Allocation Procedure
|
|
Patients were allocated to 1 of 3 groups in sequential order adopting a computer-generated (Random Allocation Software, version 1.0.0) nonstratified block randomization with varying block sizes. The trial coordinator who was not involved in patients’ outcome assessments prepared sealed opaque envelopes. Envelopes were labeled according to the study participant’s ID number, and the envelope was opened in ascending order by the study physician to determine the intervention. Neither the patients nor the study physician was blinded to the intervention.
|
|
After the baseline measurement and randomization, patients received instructions and material for the respective intervention. All groups were allowed their UC interventions except for those listed in the exclusion criteria, but they had to record all concomitant therapies and medications in the daily log.
|
|
Patients were instructed to apply CLWs once daily for 4 weeks. For the CLWs, patients were advised to take 1 to 2 raw cabbage leaves, place it on a cutting board, cut out the hard stem, and bruise the leaves using a bottle or rolling pin. The leaves had to then be layered around the knee joint, fixed with a bandage, and applied for at least 2 hours per day. It was suggested that the wraps be left on overnight. After removing the cabbage, patients had to clean the knee with warm water. In rare cases of allergic reactions—that is, swelling and itching sensation after application—patients were advised to refrain from application.
|
|
This group was provided with a 4-week supply of a TPG Voltaren (by Novartis), with its pharmacologic active agent diclofenac (10 mg/g), and advised to rub in 1 to 4 g of the gel up to 4 times daily for 4 weeks. After the study was completed, patients in this group were offered instructions on application of CLWs.
|
|
Patients in this group were advised to continue their usual activities and therapies including pain medication, but not to initiate any new therapeutic regimen for symptom management. After the study was completed, this group was offered the CLWs or the TPG as incentives.
|
|
At the assessment visit all patients rated their expectations on whether the CLW or the TPG was successful in improving their knee pain on a 100 mm VAS, with 0 mm described as “do not agree at all” and 100 mm described as “agree completely.”
|
|
Compliance was determined using the daily log. Patients were considered compliant when they followed the instructions and carried out the active interventions on 80% of the days. Compliance was used for subsequent subgroup analyses.
|
|
Current pain intensity was measured using a 0 to 100 mm VAS from the German Pain Questionnaire,15 with 0 mm indicating “no knee pain at all” and 100 mm indicating “worst knee pain imaginable.”
|
|
Functional disability was assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC).16,17 The WOMAC is a widely used standardized 24-item instrument to determine the impact of OA on daily life on 3 subscales: pain, stiffness, and physical functioning, as well as on a global score. Each item is rated on an 11-point numerical rating scale, and the final scores range between 0, indicating “no complaints or difficulties,” and 10 indicating “extreme complaints or difficulties.”
|
|
Health-related quality of life was assessed using the Short Form 36 Health Survey Questionnaire (SF-36).18 This widely used comprehensive 36-item questionnaire yields an 8-scale health profile and 2 component summaries of physical and mental health–related quality of life.
|
|
Arthritis-specific self-efficacy was measured using the German version of the Arthritis Self-Efficacy short-form Scale [arthritis self-efficacy scale (ASES-D)], an 8-item short form of the arthritis self-efficacy scale.19,20 The instrument comprises 8 items that are rated on a 10-point numerical rating scale, with 1 indicating “not confident at all” and 10 indicating “absolutely confident,” being able to control symptoms and functional disability. The ASES-D results in 1 total score constituting the average of all items.19,20
|
|
To measure physical function the 30-Second Chair Stand test was applied. Patients sat in the middle of a chair and crossed their hands on their chest. On the signal they rose to a full standing position and sat down again; they repeated this as often as possible within 30 seconds. The number of full stand-ups and pain intensity after the exercise were recorded.21
|
|
Pressure Pain Sensitivity
|
|
Patients’ pressure pain thresholds (PPT) were measured using a digital algometer (Somedic AB, Hörby, Sweden) with a 1 cm2 probe. Pressure was applied in increments of 40 kPa/s until patients indicated a perception of pain in addition to pressure. They were measured bilaterally at 3 predefined sites: over the quadriceps muscle above the patella; on the pes anserinus and the lateral joint line of the knee; and unilateral at the most painful area on the painful knee. The average of 3 measurements for each of these 7 locations was analyzed.22,23
|
|
All patients used a log to record the intensity of their knee pain (VAS), whether they carried out the prescribed interventions, and whether they took analgesics or received other OA treatments. Analgesic consumption and concomitant treatments were analyzed by frequency, and for analgesics the defined daily doses were also calculated.24
|
|
Satisfaction With Interventions
|
|
At the end of each phase, patients were asked to judge how beneficial the respective treatment was, whether they would utilize this intervention in the future, and whether they would recommend it to family and friends.
|
|
All adverse events were recorded. Patients experiencing such events were asked to see the study physician to assess their import and initiate any necessary response.
|
|
Primary and Secondary Outcome Measures
|
|
The primary outcome measure was pain intensity after 4 weeks as measured by the VAS. Secondary outcome measures included the following: pain (VAS), functional disability (WOMAC), quality of life (SF-36), and self-efficacy (ASES-D) after 4 and 12 weeks; physical function (30 s Chair Stand test) and pressure pain sensitivity (PPT) after 4 weeks; and pain intensity (VAS) and medication from the daily log, as well as compliance, satisfaction, and safety.
|
|
For the primary outcome an average pain intensity on VAS of 59.3±16.2 mm at baseline was expected, based on published data.25 The estimated minimal clinical important difference was 19.9 mm VAS. Given an effect size of d=1.045, and a 2-sided 5% level t test, 63 patients would be needed to detect this group difference with a statistical power of 90%. We planned to include 81 patients in this trial, recognizing a potential loss of analytical power due to patient withdrawal.
|
|
All analyses were based on the intention-to-treat population; that is, each patient providing baseline data was included in the final analysis. Missing data were substituted with baseline data (baseline values carried forward). The analysis of the PPT and the Chair Stand test was based on the per-protocol population only.
|
|
Baseline data comparability was ensured using the Student t test for continuous data and the [chi]2 test for categorical data.
|
|
The primary outcome was analyzed using a univariate analysis of covariance (ANCOVA), which modeled the posttreatment outcome as a function of treatment group (classified factor), and the respective baseline value (linear covariate). A stepwise gatekeeper analysis 26 was conducted to preserve the overall false-positive rate, starting with the comparison of CLW with UC, followed by CLW versus TPG. Within this model the treatment effect was estimated, accompanied with a 95% confidence interval (CI). The P-value was based on a 2-sided t test for superiority within this statistical model. For secondary outcomes the same statistical models were used, but all secondary outcomes were analyzed exploratively only. No [alpha]-level adjustments were necessary to maintain the overall type I error rate of 5%.27,28
|
|
Results from the daily log were reported explorative, and no statistical analysis was conducted.
|
|
All analyses were performed using the Statistical Package for Social Sciences software (IBM SPSS Statistics for Windows, release 22.0; IBM Corp., Armonk, NY).
|
|
From 207 patients initially screened by telephone, 115 patients were seen by the study physician, of whom 81 were enrolled. The most common reasons for excluding patients were not meeting the inclusion criteria, scheduling issues, or loss of interest in the study. Of the 81 patients enrolled, all were randomized and allocated to their intervention. During the 4-week intervention, 8 patients were lost to follow-up—3 in CLW, 1 in TPG, and 4 in UC. Reasons for dropout included loss of interest and incidence of adverse events. During the follow-up period another 2 patients were lost from the UC group because of scheduling issues and loss of interest. As all patients provided baseline data, 27 patient data sets in each group could be analyzed (see Fig. 1 for CONSORT flowchart).
|
| Opens a popup window |
Opens a popup window |
Opens a popup window |
|
|
The patients were aged 65.9±10.3 years on average; 42 women and 39 men were included (Table 1). A large proportion of patients had an education below high school level, and most were retired. Only half of the patients reported receiving prior medication for symptom management, and even fewer had received physical therapy, surgical interventions, or injections. Except for body mass index no group differences were found at baseline.
|
| Opens a popup window |
Opens a popup window |
Opens a popup window |
|
|
There were significant differences between the patients’ expectations toward the study interventions; for example, whereas the mean expectation toward CLW was 75.1±21.9 mm VAS, it was 61.2±28.3 mm for TPG (P=0.049).
|
|
Figure 2 shows the percentage of compliant patients in the active groups over the study period. Although compliance was highest at the beginning, it dropped to 80% in the last week. Altogether patients in both groups were highly compliant; 79.2% of CLW patients and 92% of TPG patients executed their respective intervention during the 4-week period on at least 80% of the days.
|
| Opens a popup window |
Opens a popup window |
Opens a popup window |
|
|
At baseline, all groups had comparable pain intensity at around 40 mm on a 0 to 100 mm VAS (P=0.824). Further analysis revealed a significant group difference between CLW and UC (difference, -12.1; 95% CI, -23.1, -1.0; P=0.033) after 4 weeks (Table 2). No group difference was found between CLW and TPG (difference, -8.6; 95% CI, -21.5, 4.4; P=0.190) (Table 3).
|
| Opens a popup window |
Opens a popup window |
Opens a popup window |
|
|
After 4 weeks, 8 patients in the CLW group, 6 in the TPG group, and 4 in the UC group showed a pain reduction equally or higher than the estimated MCID.25 After 12 weeks the corresponding numbers were 8, 6, and 5.
|
|
Secondary Outcome Measures
|
|
No difference in pain intensity between CLW and UC (difference, -7.3; 95% CI, -19.0, 4.3; P=0.210) or between CLW and TPG (difference, -7.2; 95% CI, -20.9, 6.5; P=0.290) could be found at 12 weeks.
|
|
Significant differences between the CLW and UC groups could be found for all WOMAC scales after 4 and 12 weeks (Table 2). When compared with TPG, only the subscales pain and physical function and the global score showed significant group differences at 4 weeks in favor of CLW (Table 3).
|
|
For quality of life the following effects were found (Table 2): physical functioning at 4 and 12 weeks (CLW>UC); vitality at 4 weeks (CLW>UC); and bodily pain and the physical component summary at 12 weeks (CLW>UC). Compared with TPG the following effects were found (Table 3): physical functioning, physical role functioning, bodily pain, and general health perception at 12 weeks (CLW>TPG); emotional role functioning (TPG>CLW) at 4 weeks; and the physical component summary at 4 and 12 weeks (CLW>TPG).
|
|
For PPT significant differences were found between the CLW group and the UC group for the quadriceps muscle and the pes anserinus, with higher thresholds in the CLW group, and for the quadriceps muscle when compared with the TPG group.
|
|
The test on physical function revealed no effects on the number of stand-ups, but there was an impact on pain afterward in the CLW group compared with the UC (Table 2) and TPG groups (Table 3).
|
|
When only compliant CLW patients were compared with the UC group the effect on the primary outcome remained significant (difference, -12.5; 95% CI, -24.7, -0.4; P=0.044). Compared with TPG, however, no difference was found (difference, -8.2; 95% CI,-21.5, 5.2; P=0.223). As before no effects were found at 12 weeks (CLW vs. UC, -6.2; 95% CI, -18.7, 6.4; P=0.326; CLW vs. TPG, -4.9; 95% CI, -18.6, 8.8; P=0.475).
|
|
A small but consistent decline in pain intensity was found in the CLW and TPG groups, but not in the UC group (Fig. 3A). Analysis of other drug therapies shows (Fig. 3B) that 10% to 30% of the patients used analgesics sometime during the study, with patients in the CLW tending to use fewer analgesics compared with the control group. The corresponding average daily doses of analgesics were low, between 0.00% and 0.15% of recommended daily dosage (Fig. 3C), with lowest daily doses in the CLW group. The rate of concomitant treatments was <15%, with no overall differences between the groups (Fig. 3D). The most frequently used interventions were cool pads, craniosacral therapy (TPG), heat pads, physical therapy, acupuncture, radiotherapy (CLW), mud packs, and elastic therapeutic tapes (UC).
|
| Opens a popup window |
Opens a popup window |
Opens a popup window |
|
|
Satisfaction With Interventions
|
|
Patients reported moderate benefit of both interventions after 4 weeks (CLW, 60.3±32.8 mm; TPG, 45.7±35.1 mm; P=0.188) and 12 weeks (CLW, 54.3±32.8 mm; TPG, 38.4±32.3 mm; P=0.128). After 4 weeks of use 75.0% and 70.8% of patients reported that they would consider using CLW and TPG again ([chi]2, P=0.5), and 79.2% and 75% would consider recommending CLW and TPG to family and friends, respectively ([chi]2, P=0.5).
|
|
The following adverse events were recorded during the study: 1 patient in the UC group developed bronchitis and received antibiotics. One patient in the CLW group complained of dry cough, which resolved after discontinuation of ACE inhibitor intake. One patient in the CLW group complained of itching and burning sensation during CLW application and stopped the treatment. The same patient reported a zoster infection during the trial and resigned from the study. One patient in the TPG group was diagnosed with spondylolisthesis; this patient reported back problems for several years. He received orthopedic therapy. Another patient in the TPG group was diagnosed with gastric ulceration, and even though the patient reported gastric problems over the previous 6 months a causal relationship could not be excluded. This patient resigned from further study participation. All patients were under medical treatment at their respective physicians.
|
|
This trial found that a 4-week application of CLWs was more effective than UC with respect to pain, functional disability, and quality of life. It was, however, not superior to a 4-week application of topical medication. Patients were satisfied with both interventions, and except for 2 adverse events in both groups the applications were well accepted and tolerated.
|
|
Even though CLWs are often recommended as a self-care method for knee OA, no controlled study had investigated its efficacy. Instead, only anecdotes had been published 29,30 of single patients claiming that they experienced substantial improvement.
|
|
CLWs, however, have been investigated under different conditions, such as breast engorgement during breastfeeding.12,31–33 A randomized controlled trial 32 with 120 breastfeeding women found a tendency of less breast engorgement and longer breastfeeding when using CLW compared with UC. The results of the study are, however, limited by the short application and possible baseline differences. Others 34 also found a significant relief in breast engorgement and pain after CLW, but so did those patients receiving a cold or a hot compress. A recent Cochrane review 11 concluded that evidence was insufficient to justify widespread implementation of CLWs among others.
|
|
However, CLWs are not the only herbal medicine available for OA. Several studies have investigated other topical treatments including a herbal ointment,35 ginger patches,36 or patches with traditional Chinese herbs.37,38 Conclusive evidence, however, cannot be drawn from those studies. The study by Therkleson,36 for example, compared 2 ginger preparations with each other, and no comparison with UC or a gold standard therapy was conducted. Besides herbal medicine, other topical treatments may include mud packs that according to a recent meta-analysis were effective in reducing pain in patients with knee OA.39
|
|
In the present trial CLW was superior to UC but no more effective than a TPG. It is not clear how much of the observed effects were actually due to nonspecific effects such as expectation or placebo. Further specific effects may include effects of cabbage herbal compounds such as flavonoids and glucosinolates with anti-inflammatory properties.7 Caplan 40 also referred to the drawing actions of plants (absorption); however, no study has yet investigated details of such actions. All in all effects of CLWs compared with UC were rather small but clinically relevant.
|
|
The advantages of CLWs are simplicity and low costs, and a good safety profile allowing for long-term application. Patients using CLWs also reported being very satisfied; therefore it might be recommended as a complement to conventional therapy or even an alternative when drugs are contraindicated. The application might only be discouraged in patients with allergies.
|
|
Strengths and Limitations
|
|
The strengths of the study include the randomized study design and the use of different comparators. The small number of dropouts, at least in the active intervention groups, and the overall high compliance also indicated that the tested interventions were well tolerated. Also concomitant treatments and medication were evaluated as proposed by the task force of the Osteoarthritis Research Society.41,42
|
|
Limitations include the lack of blinding of patients and physicians. Patients in the study had mild-to-moderate OA of the knee, and its validity for progressed OA can be questioned. According to the post hoc power analysis the sample size was also too small to reliably detect the actual moderate effect size on the primary outcome measure, with a power of 1-[beta] being <40%. The sample size required to determine a significant group difference of moderate size with a power of at least 80% and the same effect size found in this study would be >3 times the sample size that was actually used. However, the difference between CLWs and diclofenac was smaller than the minimal clinical important difference on which the sample size calculation was based. In further studies other test procedures should be used to determine the equality of noninferiority of interventions. Finally the use of sequential gatekeeping analyses might be discussed controversially, as those have been primarily used to test the efficacy of different drug doses in clinical trials.
|
|
Many self-care treatments for knee OA are being recommended; however, only a few have been scientifically evaluated. As active coping and patient empowerment are important parts of chronic disease management, future studies should investigate the efficacy and safety of such methods. Further studies on CLWs may include patients with more progressed OA stages, or patients with knee pain of other origins. A blinded comparison with other leaves without expected pharmacological effects might also shed light on the specificity of the effect. Last but not the least, studies using CLWs as an adjunct to systemic pain therapy could reveal additional or even dose-sparing effects of the intervention.
|
|
CLWs are more effective in the management of knee OA than UC, but not more than topical diclofenac gel. Therefore they might be recommended in patients with OA of the knee. CLWs seem safe and may be used in the longer term. However, further research is warranted
|
|
The authors express their gratitude toward the nurses of the inpatient ward for their support in developing this trial. The authors also thank the Karl and Veronica Carstens-Foundation for supporting the trial financially. The following individuals from the Department of Internal and Integrative Medicine, are also acknowledged: Jana Hochstein and Yara Lia Keldenich for help with data management, Naima Khlef for help with patient recruitment, and Kathrin Cillis, MD, who assisted in the physical examination of the patients.
|
|
1. Guccione AA, Felson DT, Anderson JJ, et al.. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–358. Bibliographic Links [Context Link]
|
|
2. Peat G, McCarney R, Croft P. Knee pain and OA in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–97. [Context Link]
|
|
3. Altman R, Asch E, Bloch D, et al.. Development of criteria for the classification and reporting of OA. Classification of OA of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. Bibliographic Links [Context Link]
|
|
4. Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford). 2002;41(suppl 1):3–6. [Context Link]
|
|
5. Bennell KL, Hunter DJ, Hinman RS. Management of OA of the knee. BMJ. 2012;345:e4934. [Context Link]
|
|
6. Pendleton A, Arden N, Dougados M, et al.. EULAR recommendations for the management of knee OA: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2000;59:936–944. Full Text Bibliographic Links[Context Link]
|
|
7. Fink MDobos G, Deuse U, Mialsen A. Hydrotherapy. Chronische Erkrankungen integrativ Konventionelle und komplementäre Therapie. München: Urban & Fischer Verlag; 2006:445–468. [Context Link]
|
|
9. Wessel A, Sekkal MKraft K, Stange R. Schmerztherapie. Lehrbuch Naturheilverfahren. Stuttgart: Hippokrates Verlag; 2010:726–752. [Context Link]
|
|
10. Brüggemann W. Kneipptherapie—ein Lehrbuch. Heidelberg, New York: Springer Verlag; 1980. [Context Link]
|
|
12. Roberts KL. A comparison of chilled cabbage leaves and chilled gelpaks in reducing breast engorgement. J Hum Lact. 1995;11:17–20. Bibliographic Links [Context Link]
|
|
14. Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414. Bibliographic Links [Context Link]
|
|
15. Korb J, Pfingsten M. Der deutsche Schmerzfragebogen—implementierte Psychometrie. Schmerz. 2003;17:S47. [Context Link]
|
|
16. Bellamy N, Buchanan WW, Goldsmith CH, et al.. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with OA of the hip or knee. J Rheumatol. 1988;15:1833–1840. [Context Link]
|
|
17. Stucki G, Meier D, Stucki S, et al.. Evaluation of a German version of WOMAC (Western Ontario and McMaster Universities) Arthrosis Index. Z Rheumatol. 1996;55:40–49. [Context Link]
|
|
18. Bullinger M, Kirchberger I. SF-36 Fragebogen zum Gesundheitszustand. Göttingen, Germany: Hogrefe; 1998. [Context Link]
|
|
19. Lorig K, Chastain RL, Ung E, et al.. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. [Context Link]
|
|
20. Mueller A, Hartmann M, Mueller K, et al.. Validation of the Arthritis Self-Efficacy Short-Form Scale in German fibromyalgia patients. Eur J Pain. 2003;7:163–171. Bibliographic Links [Context Link]
|
|
21. Gill SD, de Morton NA, Mc Burney H. An investigation of the validity of six measures of physical function in people awaiting joint replacement surgery of the hip or knee. Clin Rehabil. 2012;26:945–951. Full Text Bibliographic Links [Context Link]
|
|
23. Rolke R, Magerl W, Campbell KA, et al.. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. Bibliographic Links [Context Link]
|
|
24. WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2010. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2009. [Context Link]
|
|
25. Tubach F, Ravaud P, Baron G, et al.. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip OA: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. [Context Link]
|
|
29. Rath J. Cabbage leaf therapy of gonarthrosis. At first I smiled about white cabbage, then I tried it. MMW Fortschr Med. 2003;145:15. Bibliographic Links [Context Link]
|
|
30. Schone L. Cabbage leaf therapy of gonarthrosis. At first I smiled about white cabbage, then I tried it. MMW Fortschr Med. 2003;145:15. Bibliographic Links [Context Link]
|
|
31. Methew LA. Effectiveness of Cold Cabbage Leaves vs Hot Application on Breast Engorgement Among Postnatal Mothers in a Selected Hospital, Mangalore. Mangalore: Department of Obstetrics and Gynaecological Nursing, Sahyadri College of Nursing; 2013. [Context Link]
|
|
32. Nikodem VC, Danziger D, Gebka N, et al.. Do cabbage leaves prevent breast engorgement? A randomized, controlled study. Birth. 1993;20:61–64. Bibliographic Links [Context Link]
|
|
34. Arora S, Vatsa M, Dadhwal V. A comparison of cabbage leaves vs. hot and cold compresses in the treatment of breast engorgement. Indian J Community Med. 2008;33:160–162. Full Text Bibliographic Links [Context Link]
|
|
36. Therkleson T. Topical ginger treatment with a compress or patch for OA symptoms. J Holist Nurs. 2013;32:173–182. [Context Link]
|
|
37. Wang X, Cao Y, Pang J, et al.. Traditional chinese herbal patch for short-term management of knee OA: a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2012;2012:171706. [Context Link]
|
|
38. Wang X, Wei S, Liu T, et al.. Effectiveness, medication patterns, and adverse events of traditional chinese herbal patches for OA: a systematic review. Evid Based Complement Alternat Med. 2014;2014:343176. Full Text Bibliographic Links [Context Link]
|
|
39. Liu H, Zeng C, Gao SG, et al.. The effect of mud therapy on pain relief in patients with knee OA: a meta-analysis of randomized controlled trials. J Int Med Res. 2013;41:1418–1425. [Context Link]
|
|
40. Caplan LM. Drawing action of cabbage leaves. J Hum Lact. 1999;15:7–8. [Context Link]
|
|
41. Altman R, Brandt K, Hochberg M, et al.. Design and conduct of clinical trials in patients with OA: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage. 1996;4:217–243. Full Text Bibliographic Links [Context Link]
|
|
42. Hochberg MC, Altman RD, Brandt KD, et al.. Design and conduct of clinical trials in OA: preliminary recommendations from a task force of the Osteoarthritis Research Society. J Rheumatol. 1997;24:792–794. Bibliographic Links [Context Link]
|






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 