Indication and Clinical Profile5,6
Lurasidone is the newest atypical antipsychotic (AA) agent approved for the treatment of schizophrenia in adults. Annually, schizophrenia affects about 1% of the U.S. population aged ≥18 years. The disorder is characterized by three symptom types: positive (abnormal behavior), negative (inability to perform daily activities), and cognitive (inability to make decisions).
The development of lurasidone involved more than 40 clinical trials in about 2,700 adults. Efficacy was established by four 6-week placebo-controlled clinical studies in which lurasidone demonstrated significantly greater improvement versus placebo on the primary efficacy measures (Positive and Negative Syndrome Scale [PANSS] total score and Brief Psychiatric Rating Scale derived from PANSS).
Pharmacology and Pharmacokinetics5,6
Lurasidone is an isoindole derivative (FIGURE 3) that has a unique receptor-binding profile with high affinity for dopamine-2, serotonin- 2A, serotonin-7, serotonin-1A, and noradrenaline-2C receptors. These receptors are known to improve cognitive capabilities upon effective regulation. The drug has limited affinity for histamine-1 and acetylcholine- M1 receptors and is a partial agonist for the serotonin 5-hydroxytryptamine (5HT)1A receptor. Lurasidone enhances the functions of muscarinic acetylcholine receptors, which are known to aid in memory and learning functions.
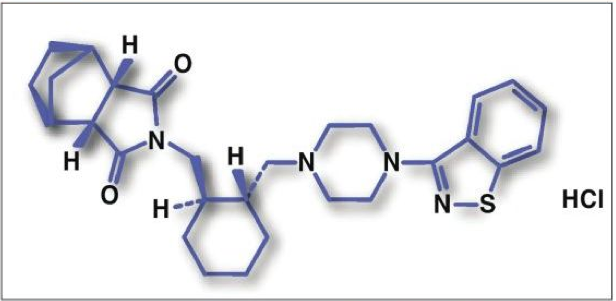
Lurasidone is 9% to 19% absorbed, producing peak serum concentrations in 1 to 3 hours. Administration with food increases both Cmax and AUC. The drug is metabolized mainly via CYP3A4 by pathways including oxidative N-dealkylation, hydroxylation of norbornane ring, and S-oxidation. Lurasidone is metabolized into two active metabolites (ID-14283 and ID-14326) and two major nonactive metabolites (ID-20219 and ID-20220). The drug is primarily cleared in the feces (80%) as metabolites.
Adverse Reactions and Drug Interactions5,6
The most common adverse reactions in clinical trials were drowsiness; akathisia; feelings of restlessness; nausea; movement abnormalities such as tremors, slow movement, or muscle stiffness (parkinsonism); and agitation. All AAs carry a black box warning alerting prescribers to an increased risk of death associated with off-label use to treat behavioral problems in older people with dementia-related psychosis. Lurasidone, like other AAs, comes with warnings for cerebrovascular adverse effects including stroke, neuroleptic malignant syndrome, tardive dyskinesias, orthostatic hypotension, hematologic abnormalities, hyperprolactinemia, seizures, cognitive impairment, suicide, and metabolic abnormalities. Lurasidone is a Pregnancy Category B drug, and use while breastfeeding should be considered only if the potential benefit justifies the potential risk to the child.
Lurasidone is not recommended for use in combination with strong CYP3A4 inhibitors or inducers. Dosage adjustment is recommended when it is used with moderate CYP3A4 inhibitors. Lurasidone is not known to interact with other CYP isozymes, Pgp, or oral contraceptives.
Dosage and Administration5,6
Lurasidone is available in 40-mg and 80-mg tablets. The recommended starting dose is 40 mg once daily, with a maximum dose of 80 mg/day. Higher doses provide no additional benefit and increase the incidence of certain adverse reactions. The dosage should not exceed 40 mg/day in patients with moderate-to-severe renal or hepatic impairment or in patients taking a moderate CYP3A4 inhibitor. Patients taking strong CYP3A4 inhibitors or inducers should not take lurasidone.
5HT機轉:圖解藥理學~23serotonin
美國食品和藥物管理局今天批准Latuda(lurasidone鹽酸)片劑用於治療成年人精神分裂症。
精神分裂症的影響約 1%的美國人口,年齡在18歲以上,在給定的一年。最突出的症狀包括幻覺,妄想,思維混亂和行為,多疑。聽聲音,其他人不聽是最常見的類型的幻覺。這些經驗可以使人們與疾病恐懼和撤回。
“精神分裂症可能是毀滅性的疾病,需要終身治療,”托馬斯說 Laughren醫師,科主任,精神病學產品在FDA的中心藥物評價和研究。 “有些病人不很好地回應某些類型的藥物治療,所以重要的是有多個可用的治療方案。”
Latuda包含在非典型抗精神病藥物類藥物。所有非典型抗精神病藥物包含黑框警告,以提醒處方者的死亡風險增加相關的標籤外使用這些藥物來治療行為問題的老年人患有癡呆症有關的精神病。沒有此類藥物被批准用於治療老年癡呆症患者的有關精神病。
四六個星期的對照研究顯示成年人與精神分裂症的有效性和安全性Latuda。在試驗中,患者治療 Latuda少了精神分裂症的症狀比那些服用非活動丸(安慰劑)。
最常見的不良反應報告的臨床試驗是嗜睡,煩躁不安和感情的衝動移動(靜坐不能),噁心,運動異常,如震顫,動作遲緩,或肌肉僵硬(帕金森病),和激動。
Latuda是製藥公司製造的Sunovion李堡,新澤西州






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 