其實臨床上就經常會有過敏並不是因為藥物本身而是藥物的賦形劑與其他無效成分造成的過敏
JAMA. 2019;321(20):1961-1962. doi:10.1001/jama.2019.4667
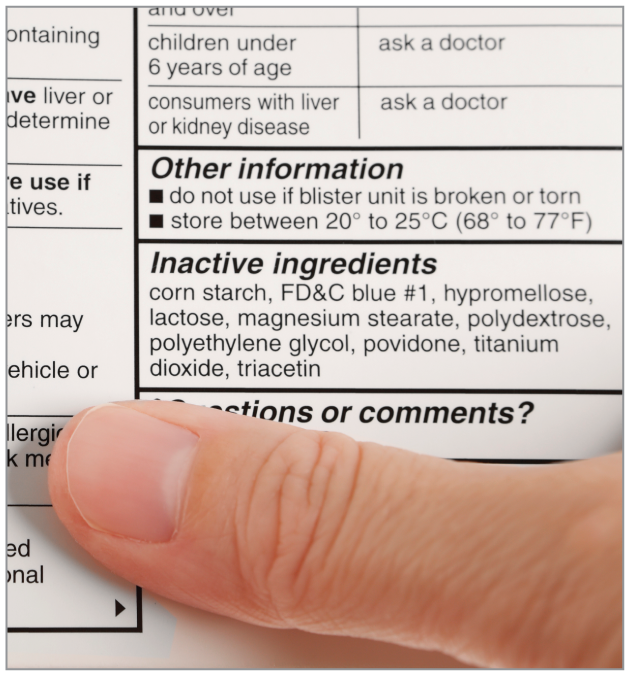
Inactive ingredients are supposed to improve a medication’s taste, appearance, absorption, or shelf life without any noticeable effect. But they aren’t always behind-the-scenes players. Some are allergens that can cause adverse events, and they’re present in nearly all prescription and over-the-counter pills and capsules, according to a recent analysis in Science Translational Medicine.
The analysis indicates that clinical reports of adverse reactions triggered by inactive ingredients are on the upswing. Some are type 1 hypersensitivity allergic reactions that produce immunoglobulin E, causing symptoms such as urticaria, angioedema, bronchospasm, or anaphylaxis.
Although severe allergic reactions are infrequent, many more people are intolerant of inactive ingredients such as lactose, which can cause gastrointestinal upset in people with insufficient lactase. The investigators found that nearly 93% of all solid oral medications contain at least 1 potential inactive ingredient—a chemical dye or peanut oil, for example—that could provoke an allergic reaction.
Several years ago, senior author and gastroenterologist Giovanni Traverso, MB, PhD, of Brigham and Women’s Hospital and the Massachusetts Institute of Technology, was involved in caring for a patient with celiac disease who was prescribed a formulation of omeprazole that contained gluten. The patient had no adverse effects, but the case motivated Traverso and his colleagues to dig into the role that inactive ingredients play in thousands of medications.
“Others have analyzed this in the past, but we wanted to look at the entire universe of formulations,” he said.
The investigators analyzed data from the National Library of Medicine’s Pillbox database, which includes information on about 354 597 inactive ingredients contained in 42 052 solid oral medications. Among the inactive ingredients, a literature search identified 38 linked with allergic reactions.
-
The analysis showed that:
-
An average tablet or capsule contains about 9 inactive ingredients.
-
Only 12% of solid oral medications contain no inactive ingredients that could affect people with allergies, intolerance, or sensitivities.
-
Only 28% of solid oral medications have at least 1 formulation that doesn’t contain any of these inactive ingredients.
-
Although 75% of people worldwide have lactose intolerance, 45% of solid oral medications contain lactose.
-
One-third of these medications contain at least 1 chemical dye such as tartrazine (FD&C [Food, Drug, and Cosmetic Act] yellow 5) that can provoke severe atopic reactions, especially in patients with existing allergies or asthma.
-
Nearly two-thirds of valproic acid capsules and 100% of progesterone capsules contain peanut oil, limiting treatment options for people with peanut allergy. However, some valproic acid formulations are made instead with corn oil to avoid allergic reactions.
-
Although lactose is the most commonly occurring allergenic inactive ingredient among those studied, others follow close behind. Corn starch is present in 37% of solid oral medications; polyethylene glycol is in 36%; povidone, 36%; carboxymethylcellulose, 21%; gelatin, 17%; brilliant blue (FD&C blue 1), 14%; sunset yellow FCF (FD&C yellow 6), 12%; allura red (FD&C red 40), 11%; propylene glycol, 11%; and indigo carmine (FD&C blue 2), 11%.
Diets low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) are recommended for people with irritable bowel syndrome or other digestive disorders. However, 55% of oral medications contain at least 1 FODMAP sugar; 5% contain more than 1. Lactose is the most commonly occurring FODMAP in solid oral medications, but others include mannitol (7%) and polydextrose (4%). Although gastrointestinal (GI) drugs often contain FODMAPs, the investigators found that most GI drug classes had FODMAP-free alternatives.
Traverso and his colleagues want to raise awareness that inactive ingredients can have clinical consequences. Even if the amount in a single drug is so low that it won’t trigger a reaction, Traverso said physicians should consider elderly patients who may take up to 10 medications a day. If nearly half of those medications contain lactose, that amount could cause adverse events.
“The key message to clinicians is to take a step back and just remember that what you’re prescribing is much more than the active ingredient,” he said.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 