Shannon A. Kavanaugh, Lisa A. White and Jill M. Kolesar
SHANNON A. KAVANAUGH, B.A., is student, School of Pharmacy, University of Wisconsin (UW), Madison. LISA A. WHITE, B.S., is student, School of Pharmacy, UW. JILL M. KOLESAR, PHARM.D., BCPS, FCCP, is Professor of Pharmacy, School of Pharmacy and Paul P. Carbone Comprehensive Cancer Center, UW
Purpose. The pharmacology, pharmacokinetics, clinical efficacy, safety, adverse effects, dosage and administration, and role in therapy of vorinostat in the treatment of cutaneous T-cell lymphoma (CTCL) are reviewed.
Summary. Vorinostat is a novel histone deacetylase (HDAC) inhibitor approved for the treatment of advanced CTCL. Its primary biochemical mechanism is to correct an aberrant balance between acetylated and deacetylated histones, the proteins involved in chromatin structure and organization. Vorinostat is metabolized and excreted following glucuronidation by the uridine diphosphate glucuronosyl-transferase (UGT) enzyme system. Polymorphisms in the gene encoding for this enzyme system, UGT1A1, may be an important predictor of vorinostat toxicity and response levels in individual patients. Vorinostat is not metabolized by and does not inhibit the cytochrome P-450 isoenzyme system, and only two drug interactions have been noted with vorinostat: warfarin and valproic acid or other HDAC inhibitors. In two Phase II studies, patients with CTCL treated with oral vorinostat demonstrated significant reductions in skin lesions and decreased disease progression. The overall response rate was approximately 30%, including one complete response and a time to response of approximately 10 weeks. At the approved 400-mg, once-daily dose, vorinostat was well tolerated, with the most common grade 1 or 2 adverse events being fatigue, nausea, and diarrhea. More-severe toxicities included thrombocytopenia, fatigue, and nausea and occurred in less than 6% of patients.
Conclusion. Vorinostat, a novel HDAC inhibitor, is efficacious and well tolerated in patients with CTCL and is being investigated for its efficacy and safety in other types of cancers and as a part of combination therapy.
Index terms: Anticoagulants; Anticonvulsants; Antineoplastic agents; Dosage; Drug administration; Drug interactions; Excretion; Lymphoma; Mechanism of action; Metabolism; Pharmacokinetics; Toxicity; Valproic acid; Vorinostat; Warfarin
Cutaneous T-cell lymphoma (CTCL) describes a group of rare and heterogeneous T-cell lymphomas primarily manifesting in the skin.1 CTCL comprises about 3.4% of all non-Hodgkin’s lymphomas (NHLs). The American Cancer Society estimated that there were 65,980 new cases of NHL in the United States in 2009; therefore, approximately 2,250 new cases of CTCL are diagnosed nationwide each year.2 CTCL is caused by proliferation of mature T cells that invade the skin and is commonly characterized as mycosis fungoides or Sézary syndrome. Mycosis fungoides, the most common form of CTCL, progresses slowly and manifests as scaly patches, plaques, or tumors on the surface of the skin, with accompanying pruritus. Sézary syndrome is a more aggressive form of CTCL and may be characterized as a progression of mycosis fungoides but can occur de novo. Characteristics of Sézary syndrome include erythrodermas and cutaneous tumors, as well as circulating malignant T cells.1
Prognostic indicators of CTCL include the level of skin involvement and the level of lymph node or visceral involvement, as described by the stages of CTCL.3 Stage Ia CTCL is characterized as less than 10% of the skin’s surface covered with plaques or patches. In stage Ib, 10% or more of the skin’s surface is covered with plaques or patches. Stage IIa CTCL involves any amount of skin and enlarged lymph nodes. Stage IIb differs only in the presence of one or more cutaneous tumors. A diagnosis of stage III CTCL implies reddening of the majority of the skin and the presence of cutaneous lesions. In stages IIa–III CTCL, lymph nodes may be enlarged, but cancer has not spread to them. In stage IVa CTCL, skin appears reddened and cutaneous lesions (as described in stage III) are present with cancer spread to the lymph nodes. Finally, stage IVb CTCL indicates skin reddening, cutaneous lesions, and the spread of cancer to other organs. The most commonly affected organs are the lungs, the spleen, and the liver.1 The most important factors associated with a positive disease outcome are limited skin involvement and a complete response to therapy.3
The type and extent of therapy suggested for CTCL are based on the clinical stage of the disease, the prognosis, and the input from the patient pertaining to quality of life. For early-stage CTCL with limited skin involvement, topical therapy with corticosteroids is the preferred initial management.1 This can be combined with mechlorethamine, carmustine, and topical retinoids such as 13-cis retinoic acid and tazarotene. Psoralen plus ultraviolet A or total-skin electron beam therapy may also be effective alone or combined with other topical therapies. Patients with more-extensive disease typically require systemic therapy. Bexarotene or interferon may be used as a first-line systemic therapy. Use of arabinosylguanine, forodesine, fludarabine, chlorodeoxyadenosine, denileukin diftitox, oral methotrexate, doxorubicin, gemcitabine, etoposide, pentostatin, bortezomib, cyclophosphamide, vincristine, or prednisolone may also be considered as monotherapy or in various combinations for patients progressing after first-line treatment. Though many of these treatments are effective, most CTCL patients rapidly become refractory to conventional agents.1
As a result, novel drugs have been developed that target epigenetic abnormalities associated with CTCL. One such medication is vorinostat (Zolinza, Merck and Co.), which received marketing approval from the Food and Drug Administration (FDA) in 2006 for the treatment of CTCL in patients with progressive, persistent, or recurrent disease who were receiving or after receiving two systemic therapies.4 This article reviews the pharmacology of vorinostat and findings from important vorinostat clinical trials that led to its approval as a treatment for CTCL.
Chemistry
The chemical name of vorinostat is N-hydroxy-N'-phenyloctanediamide; vorinostat has an empirical formula of C14H20N2O3 and a molecular weight of 264.32 g/mol.5 The pKa of vorinostat is approximately 9. Vorinostat is slightly soluble in water, alcohol, isopropanol, and acetone and is completely soluble in dimethyl sulfoxide.
Pharmacology
Vorinostat is a member of the hydroxamic acid subclass of histone deacetylase (HDAC) inhibitors.6 Its primary biochemical mechanism is to correct an aberrant balance between acetylated and deacetylated histones, the proteins involved in chromatin structure and organization. Appropriate histone acetylation and deacetylation, catalyzed by histone acetyltransferases (HATs) and HDACs, respectively, is crucial for regulating gene expression.7 Histones that have been acetylated by HATs have a neutral charge and, therefore, a low affinity for negatively charged DNA, causing the chromatin structure to relax and transcription to occur. Conversely, when histone acetylation is reversed by HDACs, the positively charged histones coil DNA tightly, disabling transcription.
Certain cancers, especially those of hematologic and epithelial origin (e.g., CTCL), are associated with hypoacetylation, which causes a decrease in the expression of genes responsible for cell differentiation, cell-cycle control, apoptosis, and tumor suppression.8 The binding of vorinostat to the active site of specific classes of HDACs inhibits the enzymes’ activity, inducing hyperacetylation of histones and the transcription of genes responsible for preventing carcinogenesis and tumorogenesis (Figure 1).9
Vorinostat is relatively selective toward cancer cells. This suggests that while the pharmacology of vorinostat is centered on its roles as a regulator of gene transcription, it may exert its therapeutic effects through other indirect mechanisms. Some of these suggested mechanisms include alteration of microtubule functioning or angiogenic signaling and stimulation of an immune response through expression of major histocompatibility complex antigens on tumor cells.10
Pharmacokinetics
Pharmacokinetic studies of orally administered vorinostat doses of 200–600 mg have demonstrated a linear relationship between the plasma concentration of vorinostat and dose.11 The mean ± S.D. maximum plasma concentration (Cmax) is 658 ± 439 ng/mL, with a median time to reach Cmax (tmax) of 106 minutes. The mean ± S.D. area under the concentration–time curve (AUC), half-life (t ), and apparent clearance (CLapp) are 101,856 ±105,570 ng/mL·min, 88.9 ± 20.5 minutes, and 4,409 ± 2,682 mL/min, respectively. The Cmax and AUC increase proportionally with increased dosage, while the tmax, t
), and apparent clearance (CLapp) are 101,856 ±105,570 ng/mL·min, 88.9 ± 20.5 minutes, and 4,409 ± 2,682 mL/min, respectively. The Cmax and AUC increase proportionally with increased dosage, while the tmax, t , and CLapp remain constant. A fasting bioavailability of 43% indicates very reasonable absorption of vorinostat into the bloodstream. The absorption and bioavailability of vorinostat do not significantly differ in the absence or presence of food, although administering vorinostat with food may prevent some gastrointestinal discomfort.
, and CLapp remain constant. A fasting bioavailability of 43% indicates very reasonable absorption of vorinostat into the bloodstream. The absorption and bioavailability of vorinostat do not significantly differ in the absence or presence of food, although administering vorinostat with food may prevent some gastrointestinal discomfort.
Vorinostat is metabolized and excreted after glucuronidation by uridine diphosphate glucuronosyltransferase (UGT). Polymorphisms in the gene encoding for this enzyme system, UGT1A1, may be an important predictor of vorinostat toxicity and response levels in individual patients. A variety of pharmacogenomic studies of other UGT-metabolized chemotherapeutic agents have identified specific polymorphisms that confer decreased UGT activity, with an accompanied increase in adverse effects and decrease in response.12 Similarly, certain polymorphisms in the thymidylate synthase gene may predict whether vorinostat will generate an efficacious response.13 Studies are being conducted to further determine the extent to which these polymorphisms affect the safety and efficacy of vorinostat.14
Clinical efficacy
An open-label, single-site, single-group, nonrandomized study was conducted to assess the safety and efficacy of oral vorinostat in 33 patients with CTCL that was intolerant or refractory to conventional topical or systemic treatments.15 CTCL stages represented in this study ranged from Ia to IVb. The patients were divided into three groups. Group 1 received a 400-mg, once-daily dosage of vorinostat. Group 2 received vorinostat 300 mg twice daily for three days per week for four weeks, followed by five days per week for the remainder of the study. Group 3 received vorinostat 300 mg twice daily for two weeks, followed by seven days without the drug and then 200 mg twice daily for the remainder of the study.
Response to vorinostat therapy was measured by changes from baseline in the proportion of skin patches, plaques, or tumors to the total body surface area; lymph node size; extent of blood involvement; and pruritus. Pruritus was rated on a scale of 0–10 by the patient before and throughout treatment. Complete response to vorinostat therapy was defined as a 100% reduction in skin lesions after four weeks of therapy, and a partial response required a reduction of  50% in skin lesions or lymph node size and blood involvement four weeks into therapy. Stable disease was defined as no change from baseline after eight weeks of treatment, and progressive disease was defined as a 25% or greater increase in symptoms.
50% in skin lesions or lymph node size and blood involvement four weeks into therapy. Stable disease was defined as no change from baseline after eight weeks of treatment, and progressive disease was defined as a 25% or greater increase in symptoms.
This study found an average time to response of 11.9 weeks and an overall response rate of 24.2%; all responses were partial. Groups 1, 2, and 3 had response rates of 31%, 9%, and 33%, respectively. A 23% response rate was observed in patients previously treated with bexarotene, though this was not a requirement for participation. The average time to progression was 30.2 weeks. A reduction in pruritus from baseline was reported by 45% of participants.
Another Phase II study of oral vorinostat provided the pivotal evidence for FDA’s approval of the drug’s labeling.16 This open-label, multisite, single-group, nonrandomized study evaluated the efficacy of vorinostat in 74 patients with CTCL who had tried two or more prior systemic therapies, one of which was bexarotene. CTCL stages ranged from Ib to IVb. Each participant received 400 mg of vorinostat seven days per week. Dosage modifications, due to drug toxicity, included a dosage reduction to 300 mg seven days per week and, if necessary, to 300 mg for five days per week. Criteria for evaluating response to treatment were the same as those in the previously described study.15
The reported time to response from this study was 7.9 weeks. The overall response rate was 29.7%, and one patient had a complete response. This rate was very similar to the response rate of group 1 in the Phase II(a) study (31%),15 who received the same 400-mg, once-daily dosage. No average time to progression was reported, but values ranged from 11.1 weeks to over 67.1 weeks. A reduction in pruritus from baseline was reported by 32% of participants. Although pruritus relief was significant in both trials, it was not included in the FDA-approved indications due to the lack of a control group and the lack of blinding in these studies.
Safety
Toxicities were observed more frequently with dosing regimens exceeding 400 mg daily, rendering the clinical benefits for higher dosing minimal. The following discussion of adverse events is consistent with an oral regimen of 400 mg once daily, the maximum FDA-approved dosage.4
The most common adverse effects of vorinostat were fatigue (n = 45, 52.3%), diarrhea (n = 45, 52.3%), and nausea (n = 35, 40.7%), with the majority being grade 1 (mild, no intervention indicated) or grade 2 (moderate, noninvasive intervention indicated).17 The most common grade 3 (severe or disabling, hospitalization indicated) or grade 4 (life-threatening, emergency intervention indicated) adverse effects were thrombocytopenia (n = 5, 5.8%), fatigue (n = 3, 3.5%), and nausea (n = 3, 3.5%). Serious adverse events included pulmonary embolism (n = 4, 4.6%), squamous cell carcinoma (n = 3, 3.5%), and severe anemia (n = 2, 2.3%).4 As discussed previously, UGT1A1 polymorphisms may play a role in the extent to which certain patients experience some of these adverse effects. There have also been reports of QTc-interval prolongation in some patients taking vorinostat.18 Patients with a history of QTc-interval prolongation or those taking antiarrhythmic medications should undergo regular electrocardiographic screenings. Other common laboratory abnormalities include increased serum glucose and creatinine concentrations and proteinuria.18
Vorinostat is not metabolized by and does not inhibit the cytochrome P-450 (CYP) isoenzyme system; therefore, drug–drug interactions are not anticipated for other medications metabolized by CYP.5 Despite this, two specific drug interactions with vorinostat have been documented.5 First, patients taking warfarin derivatives have a higher risk of bleeding due to prolonged prothrombin time and International Normalized Ratio. Second, patients taking valproic acid or other HDAC inhibitors may develop severe thrombocytopenia and bleeding of the gastrointestinal tract.
Vorinostat is classified as a pregnancy category D drug.5 No formal studies have been conducted in women to determine the effects of vorinostat on the developing fetus.18 However, a study in animals found that vorinostat crosses the placenta and may harm the developing fetus.19 The most common developmental effects observed were low fetal birth weight and incomplete ossification of the skull, vertebrae, and other bones of the axial skeleton. No formal clinical trials have been conducted to investigate other possible interactions or contraindications.
Dosage and administration
The approved dosage of vorinostat is 400 mg orally once daily.4 The 400-mg daily dosage was associated with a response rate of approximately 31% and few life-threatening adverse effects. In contrast, the 300-mg twice-daily dosage produced an overall response rate of only 21%, with patients experiencing a greater number of grade 3 and 4 pulmonary embolisms and thrombocytopenia. For some patients, a reduction of the dosage to 300 mg once daily may be warranted if adverse effects become intolerable.
Vorinostat is supplied as 100-mg capsules approved for oral administration; however, an i.v. formulation has been comparatively analyzed for efficacy and safety.20 Response rates for i.v. vorinostat, given 300–600 mg/m2 for five days per week for three weeks, were comparable to those with the oral dosage of 400–600 mg daily; however, severe hematologic toxicities were regularly observed in the group receiving i.v. therapy. This additional adverse effect and the inconvenience of daily infusions render the oral formulation better suited for CTCL treatment.
To date, no formal studies have been conducted to establish dosage adjustments for patients with renal or hepatic impairment. Nonetheless, patients with hepatic impairment should be monitored closely for toxicities.5 Patients with renal impairment should also be monitored to ensure proper removal of the drug’s inactive metabolites.
Role in therapy
Various other skin-directed therapies and cytotoxic chemotherapies for managing CTCL may be considered as options for patients with systemic and progressive disease before using vorinostat.1 So far, no definitive clinical trials have compared the effectiveness of vorinostat to that of other CTCL therapy options. Comparative studies are unlikely to be performed because CTCL is a relatively rare disease. However, since the severe adverse effects of cytotoxic chemotherapy are well-known, vorinostat’s relative safety may provide a quality-of-life advantage for patients with CTCL.
Conclusion
Vorinostat, a novel HDAC inhibitor, is efficacious and well tolerated in patients with CTCL and is being investigated for its efficacy and safety in other types of cancers and as a part of combination therapy.
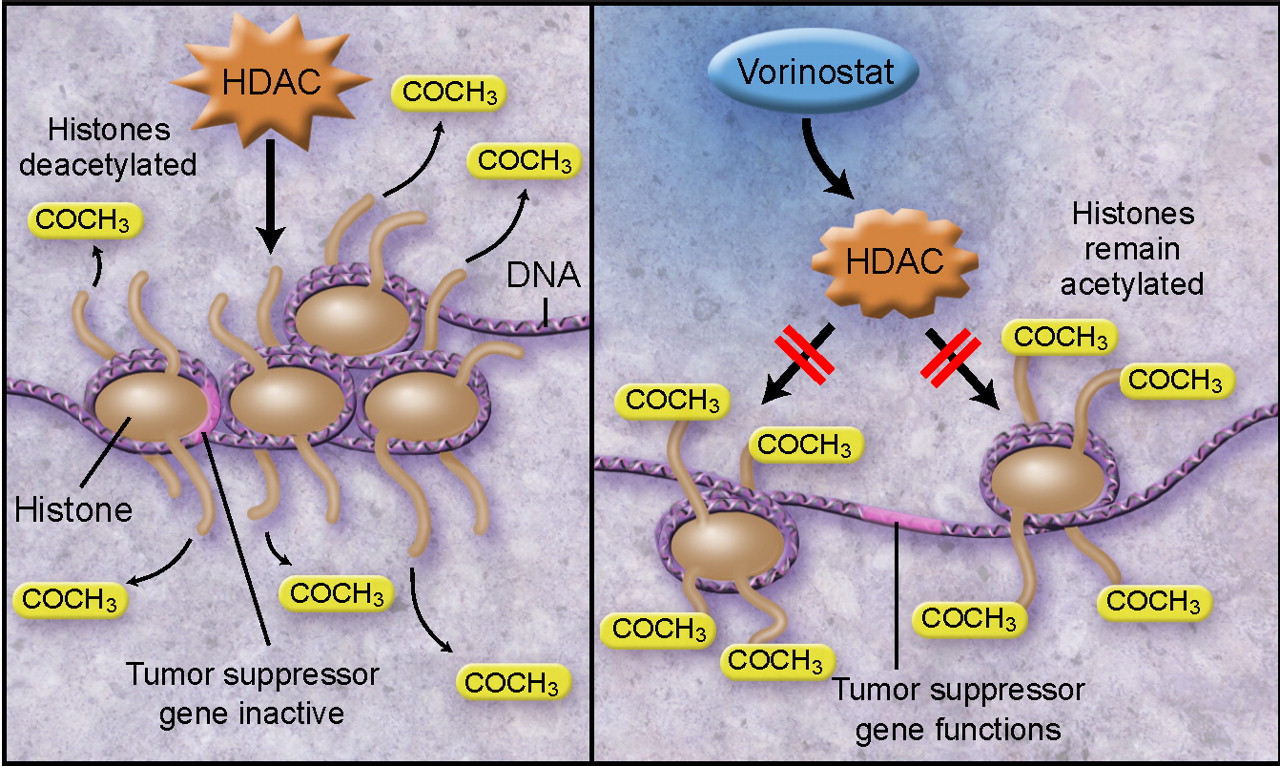
Figure 1 Mechanism of action of vorinostat. The tight coiling of DNA that results from the deacetylation of histones inhibits the transcription of important tumor suppressor genes (left). With histone deacetylases (HDACs) inhibited, acetylated histones cause DNA relaxation, allowing tumor suppressor genes to be accessible for transcription (right). Illustrated by Taina Litwak, CMI.
藥罐就是長這樣:
想多了解一點機轉:圖解藥理學 13 化療藥物機轉08





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 