September 12, 2019
N Engl J Med 2019; 381:1046-1057
DOI: 10.1056/NEJMra1800345
Asthma and hypertension are common chronic diseases, each with attendant morbidity, mortality, and economic effects. It is estimated that 300 million people worldwide have asthma, and an increase in prevalence to 400 million is anticipated by 2025. Approximately 250,000 asthma-related deaths occur yearly, many of which are believed to be avoidable.1 In the United States, more than 8% of adults have asthma, with disproportionate representation among women, ethnic minorities, and people who are economically disadvantaged. Asthma-related health expenditures in 2013 were estimated at $80 billion.2 The presence of hypertension with asthma creates an additional health burden; hypertension is the world’s most common modifiable risk factor for cardiovascular disease and death. Worldwide estimates suggest that 874 million adults have a systolic blood pressure higher than 140 mm Hg,3,4 and the prevalence of hypertension, like that of asthma, is increasing, along with costs, morbidity, and mortality.5 Elevated systolic blood pressure was the leading global contributor to preventable death in 2015.6 In the United States, approximately one in three adults has high blood pressure.7 Since current hypertension guidelines incorporate new evidence that has led to lower thresholds for treatment, the number of persons considered to have hypertension will expand, and the attendant costs will escalate.3
Patients with asthma are more likely to have hypertension than those who do not, independent of traditional risk factors.8 A diagnosis of hypertension is associated with augmented asthma severity,9and reduced lung function has been correlated with heightened cardiovascular mortality.10 Given the bidirectional relationship between compromised lung function and compromised cardiovascular function, the rationale for treating and controlling hypertension in persons with asthma is compelling. Although the effect of blood-pressure control on asthma is largely unexplored, the risk of death from cardiovascular disease is decreased when systolic blood pressure is reduced to levels below 130 mm Hg.11-14 In this review, we discuss the potential mechanistic links between hypertension and asthma, the influence each condition has on the other, and approaches to the treatment of hypertension in adult patients with asthma.
Mechanistic Relationship between Hypertension and Asthma
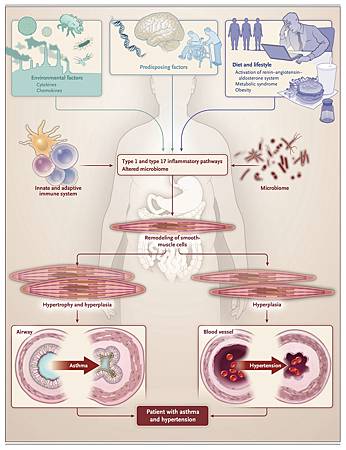
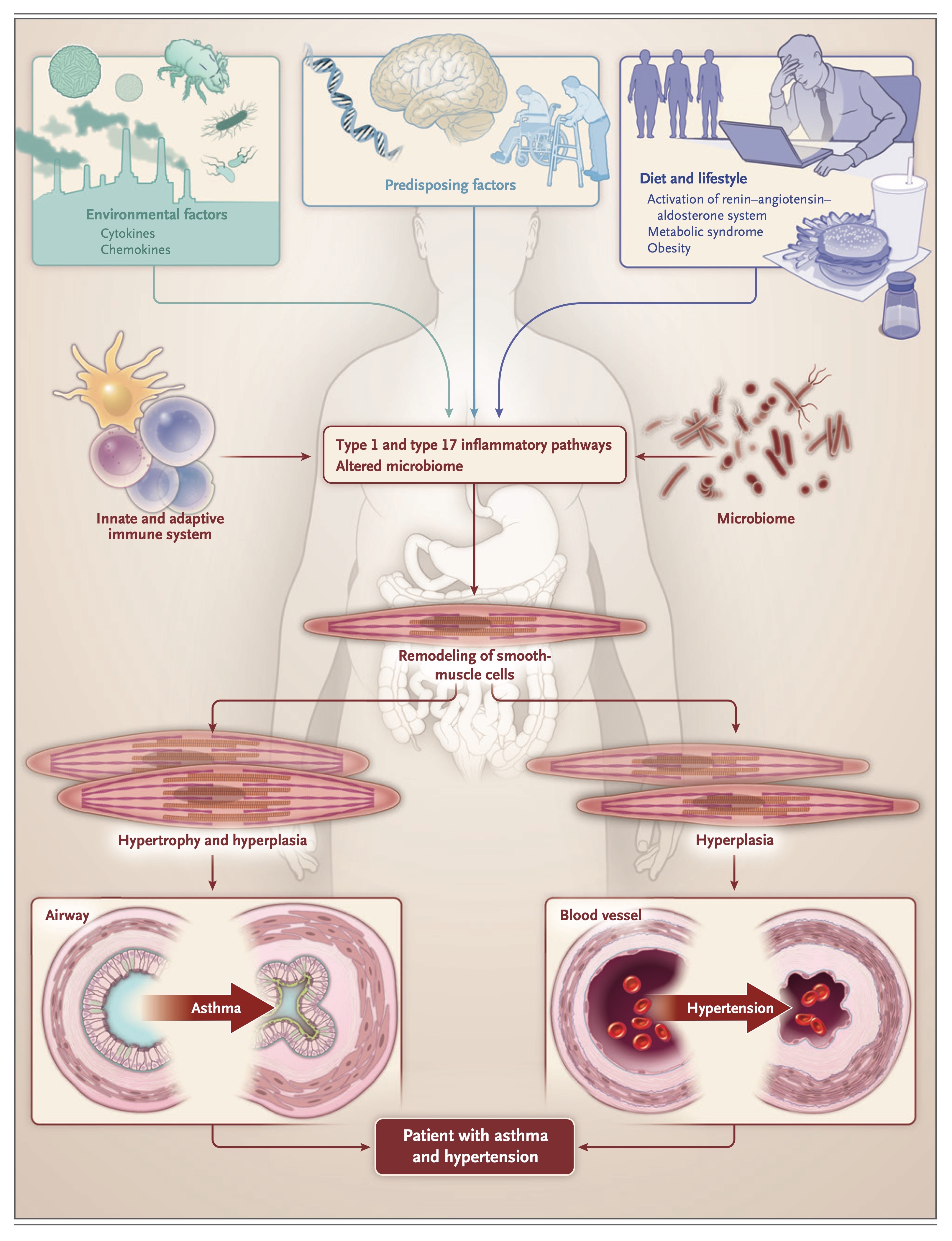
Predisposing factors (genetic profile, stress, and age), dietary and lifestyle choices, and inflammatory mechanisms all contribute to the hypertensive asthmatic phenotype. These factors may be important in understanding the development of the condition (Figure 1).
Systemic inflammation serves as an underpinning for the burden of disease that accrues from hypertension and asthma.15 Inflammation is widely accepted as being both fundamental to the pathogenesis of asthma16 and central to the development of hypertension and its deleterious consequences.17,18 A large cross-sectional study of middle-aged persons with asthma showed that the prevalence of hypertension was higher among those with lower values for forced expiratory volume in 1 second (FEV1), with the risk of hypertension increasing as the FEV1 decreased.19 Furthermore, C-reactive protein levels, a marker of systemic inflammation related to interleukin-6 and hypertension, were correlated with the rate of loss of FEV1.20 Such findings may imply a reciprocal relationship, wherein systemic inflammation influences the disease course for both hypertension and asthma.
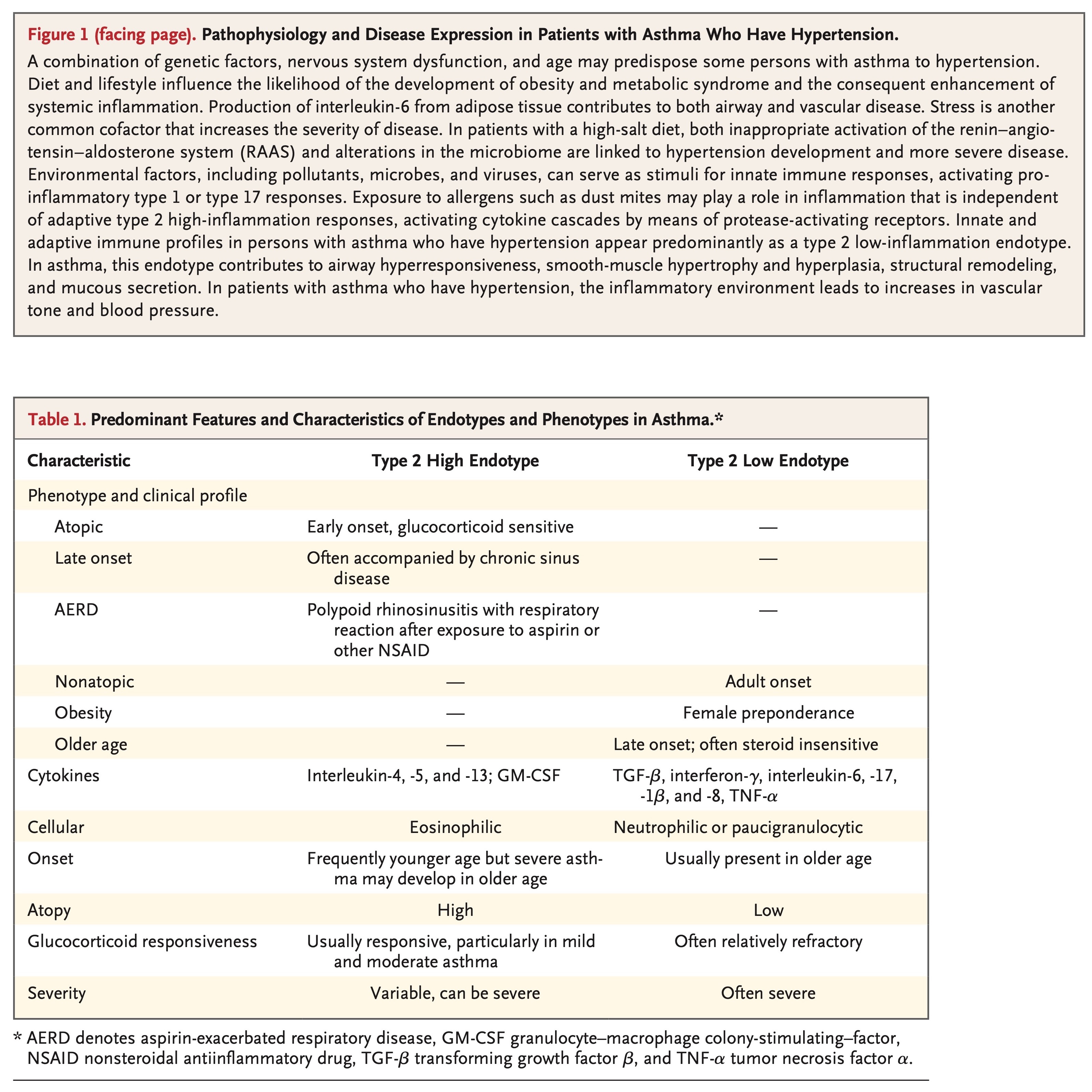
Asthma is currently understood as a disorder that is characterized by two main endotypes: type 2 high inflammation and type 2 low inflammation. These subtypes are broadly defined by their predominant underlying mechanism, which is largely determined by the T cells or innate lymphocytes and cytokines that are involved.21 Each endotype can be further subdivided into multiple phenotypes that are distinguished by clinical features, pathological findings, and biomarkers (chemokines) (Table 1). Owing to the lack of uniform criteria for classifying types of asthma, estimates of the prevalence of type 2 high- and type 2 low-inflammation endotypes vary; however, each endotype appears to represent approximately half the population with asthma.16
The Severe Asthma Research Program conducted multiple studies in which patients with asthma were grouped into discreet clusters on the basis of a hierarchical analysis of variables that included clinical characteristics, biomarkers, cellular profiles, lung function, atopic status, responses to treatment, gene expression, and coexisting conditions.22-24 It is notable that two studies that included hypertension as a variable showed cosegregation of hypertension with asthmatic profiles that are typical for the type 2 low-inflammation endotype.22,23 Patients with features of the type 2 low-inflammation endotype (older age, later onset of asthma, higher body-mass index [BMI], greater severity of disease, and low atopy) were also more likely to have hypertension (48 of 175 patients [27%]) than patients with the type 2 high-inflammation endotype (50 of 551 patients [9%]).22 The results of a separate study based on clinical characteristics and assessments of inflammatory cells in blood and sputum showed a significantly higher incidence of hypertension in clusters distinguished by severity of disease, older age, later onset of disease, higher BMI, and greater resistance to treatment with glucocorticoids: 31% (51 of 164 patients) as compared with 11% (28 of 259 patients).23 These observations suggest that type 2 low inflammatory pathways may provide a pathogenic mechanism that links these two diseases.
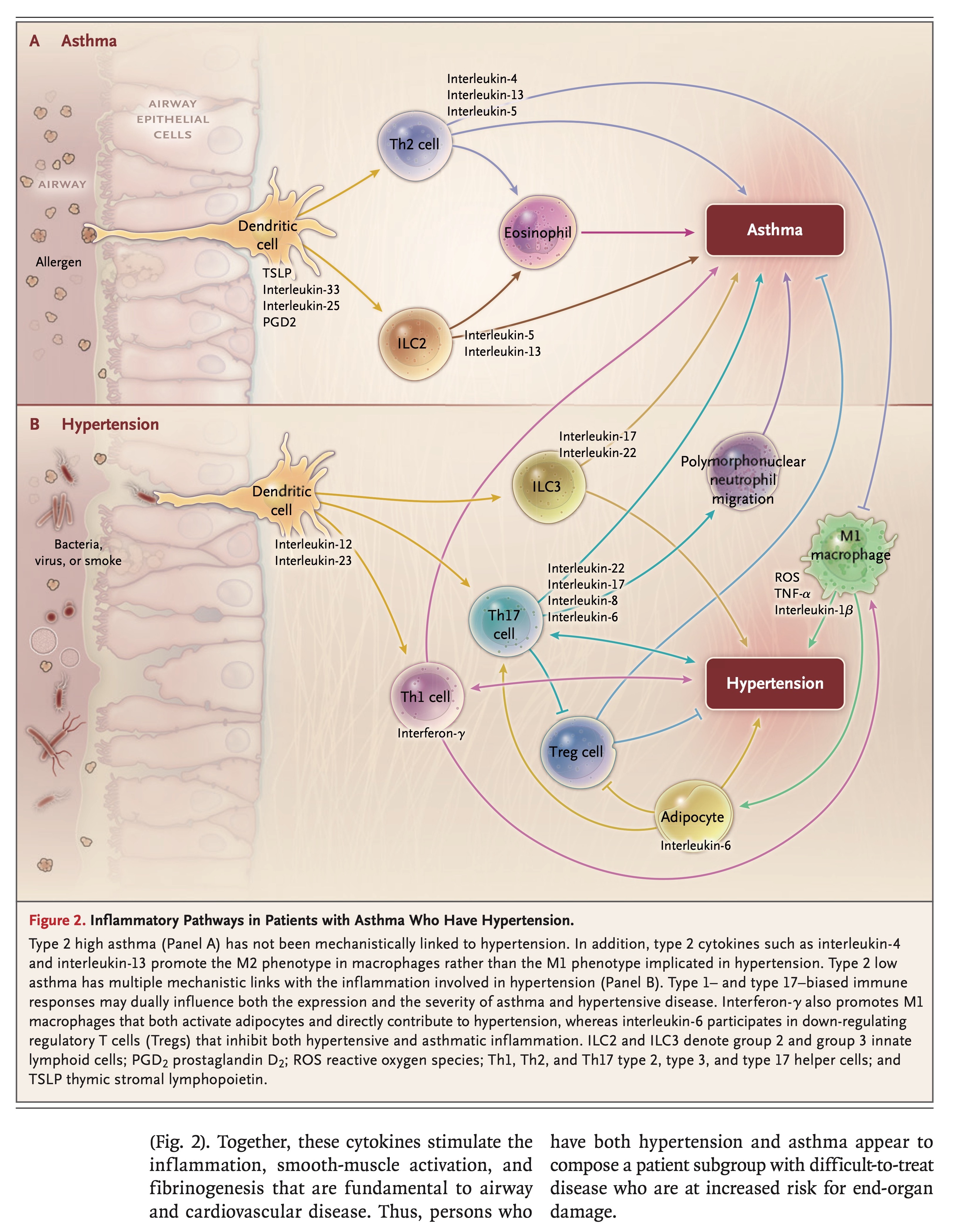
The degree of inflammation in patients with hypertension and asthma reflects the conjoint effect of both conditions. Hypertension skews T cells toward a proinflammatory (type 1 helper T-cell [Th1 cell]) phenotype, characterized by increased interferon-γ responses and decreased type 2 helper T-cell (Th2 cell) responses.25 In asthma, enhanced airway hyperresponsiveness and severe disease are associated with elevated levels of interferon-γ.26 Correspondingly, in hypertension, interferon-γ and Th1-cell polarization contribute to blood-pressure elevation and its sequelae.17
Interleukin-17 has also been shown to play a major role in the development of hypertension and its related end-organ damage in both studies in animals and in vitro models. Interleukin-17 induces a proinflammatory phenotype in vascular smooth-muscle cells by enhancing the release of mediators, including interleukin-6, CXCL8 and CXCL10, and C-reactive protein.27 Both anti–interleukin-17 treatment and genetic deletion have been shown to reduce hypertension in studies in animals.17,27 A role for interleukin-17 is also evident in some patients with severe asthma in whom an elevated level of interleukin-17 is correlated with neutrophil infiltration, airway hyperresponsiveness, and a lack of sensitivity to glucocorticoids. In these patients, interleukin-17 is capable of inducing secretion of proinflammatory cytokines from lung structural cells and airway smooth muscle, including tumor necrosis factor α, interleukin-1β, granulocyte colony-stimulating factor, and interleukin-6 as well as the chemokines CCL11 (eotaxin) and CXCL8 (interleukin-8), which are important in airway inflammation and remodeling.28,29 Surprisingly, in one trial, the targeting of interleukin-17 failed to ameliorate symptoms in patients with severe asthma30; however, a subgroup analysis identified patients with highly reversible depression in FEV1 who had some improvement, as reflected by the Asthma Control Questionnaire. The functional role of interleukin-17 in the contraction of smooth-muscle cells may offer a partial explanation for the more favorable response in this subgroup.31
Experimental evidence supports the concept that elevated interleukin-6 levels can drive the differentiation of CD4+ T cells through interaction with transforming growth factor β to promote skewing toward type 17 helper cells (Th17) cells, leading to a reduction of regulatory T cells (Tregs). Tregs play a protective role in the development of hypertension that is related in part to production of interleukin-1027 and play a critical role in the regulation of asthma development.32
Interactions among interferon-γ, interleukin-17, and interleukin-6 have the potential to affect disease expression for both hypertension and asthma (Figure 2). Together, these cytokines stimulate the inflammation, smooth-muscle activation, and fibrinogenesis that are fundamental to airway and cardiovascular disease. Thus, persons who have both hypertension and asthma appear to compose a patient subgroup with difficult-to-treat disease who are at increased risk for end-organ damage.
OBESITY AND METABOLIC DYSFUNCTION
Elevated interleukin-6 levels and systemic inflammation result in metabolic dysfunction that increases the morbidity associated with both hypertension and asthma. Secretion of proinflammatory cytokines, notably interleukin-6, by adipocytes and inflammatory macrophages in white adipose tissue is of pathogenic importance in asthma,33 a condition in which interleukin-6 appears to be a biomarker for metabolic dysfunction and severe disease.34 Elevation of interleukin-6 levels has been identified in association with hypertension in studies in humans and animals, in which it has been shown to be related to disease development.35,36
SMOOTH-MUSCLE REMODELING AND VASCULAR BIOLOGY
Smooth-muscle remodeling, driven in substantial part by inflammatory cytokines, is a critical component of both asthma and hypertension.37-39 Hyperplasia and abnormal contracture of the smooth-muscle cells surrounding the airway play an important role in airway obstruction in asthma. Similarly, the abnormal contraction and proliferation of smooth-muscle cells are well recognized as features of the vascular remodeling and endothelial abnormalities associated with hypertension.
Genetic factors clearly affect the expression of asthma and hypertension; however, the relevant interrelations are complex and difficult to dissect. Polymorphisms in β-adrenergic receptors on smooth-muscle cells have been reported in patients with both hypertension and asthma, but their importance in disease manifestation may lie in their modulation of the response to antagonists and agonists, which could have more influence on treatment outcomes than on disease causation.40 In contrast, modification of the vascular smooth-muscle cell phenotype in response to local environmental influences is thought to play a critical role in the pathogenesis of hypertension and asthma as well as atherosclerosis.41
DIETARY SALT
Dietary salt intake has long been considered to be relevant to the development of cardiovascular conditions. Early observations associating higher salt intake with blood-pressure elevation have been refined to identify subpopulations of salt-sensitive and salt-insensitive persons.42 The responsiveness of the kidneys, sympathetic nervous system, and vasculature to salt has been observed.43 The immune system may be important in responsiveness to salt. For example, Th17 cells appear to play a role in mediating the relationship between salt exposure and hypertension.17,18,27 In a mouse model, high-salt diets promoted the generation of Th17 cells through alterations in the gut microbiome, with depletion of Lactobacillus murinus. Salt-sensitive hypertension is prevented by treatment with L. murinus, which has a modulating effect on Th17 cells. Evidence suggests that the effect is related to the tryptophan metabolites produced by this organism.44 Healthy persons on a high-salt diet are also reported to have reduced levels of lactobacillus, elevated levels of Th17 cells, and elevated blood pressure, all of which support the proposition that higher salt intake plays a role in inflammation in humans.44-46 At present, similar information related to dietary salt content in patients with asthma is lacking.
Management of Disease in Patients with Asthma and Hypertension
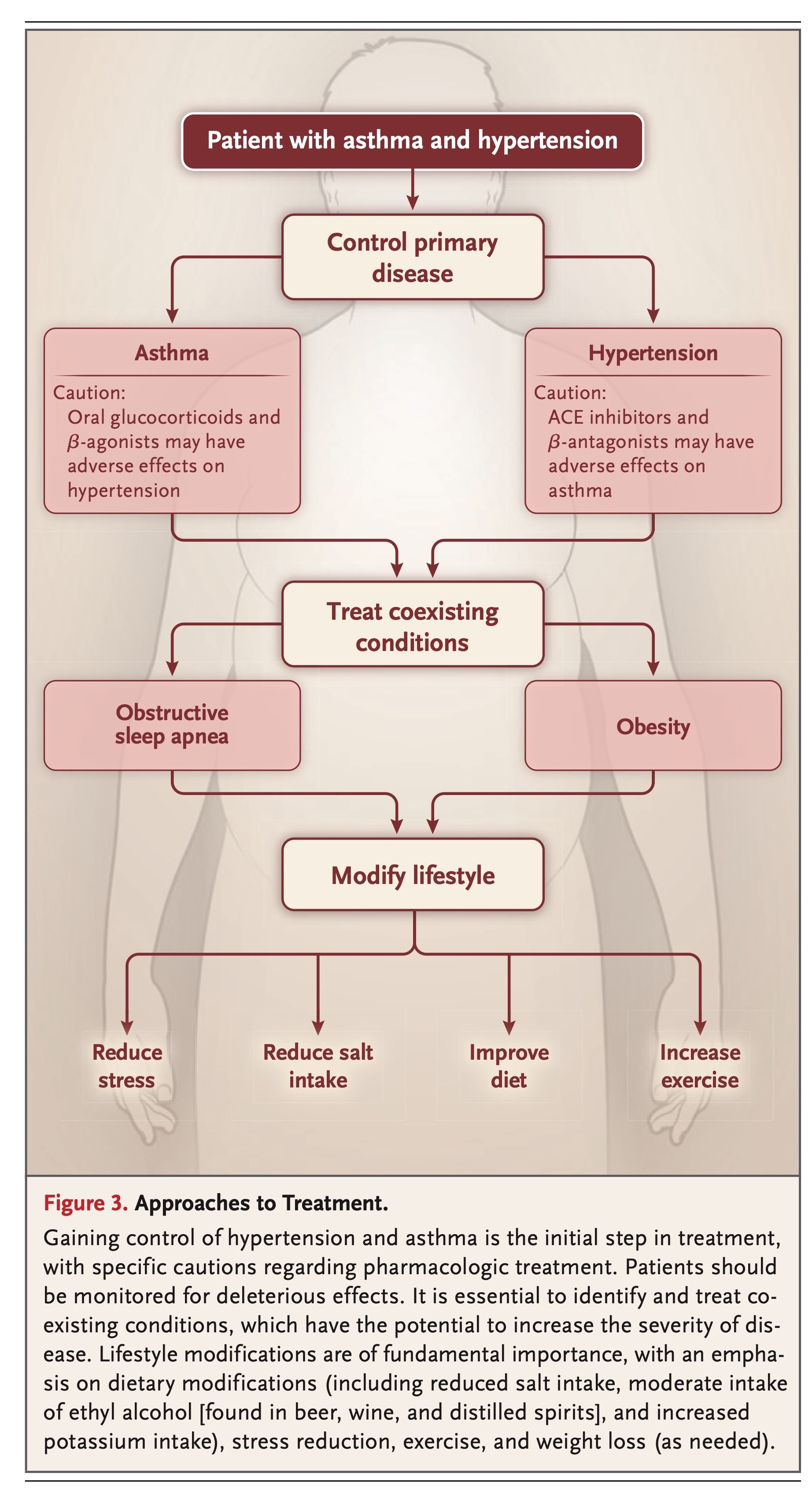
The mechanisms linking asthma and hypertension not only are of theoretical interest but also have implications for therapy and disease management. The treatment of hypertension in a patient with asthma should involve a multifactorial approach that involves control of both conditions, treatment of coexisting conditions, and the institution of lifestyle modifications (Figure 3).
PHARMACOLOGIC TREATMENT OF HYPERTENSION IN PATIENTS WITH ASTHMA
In the 2017 report of the American College of Cardiology–American Heart Association Task Force on clinical practice guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults, hypertension was categorized as either stage 1 (130–139/80–89 mm Hg) or stage 2 (>140/>90 mm Hg).3Pharmacotherapy was recommended for patients who have or who are at high risk for cardiovascular disease at stage 1 and for all patients at stage 2. It is noteworthy that the threshold for a diagnosis of hypertension was lowered in the 2017 guidelines, a development that has led to substantial controversy, since it has expanded the percentage of adults in the United States with a diagnosis of hypertension from 32% to 46%.
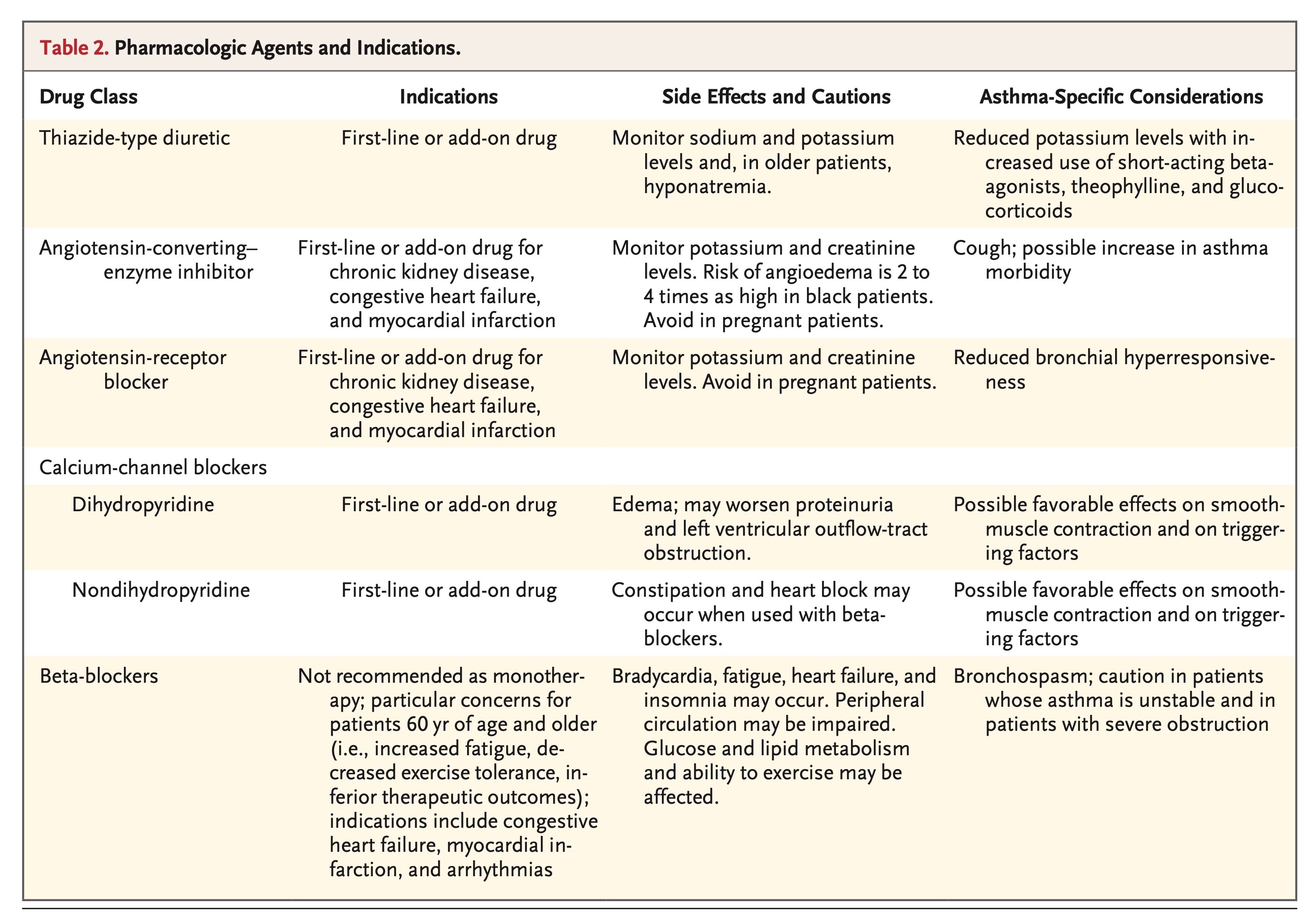
What does the treatment of hypertension generally accomplish, and how might that treatment affect patients with asthma? To date, all-cause mortality has not differed significantly among patients with hypertension treated with any of the four major classes of first-line antihypertensive agents (Table 2). The degree of blood-pressure reduction rather than the choice of antihypertensive medication appears to be the major determinant of outcome.47 In patients with asthma, however, additional issues related to various pharmacologic agents should be considered.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 