DRUGDEX® Evaluations
COENZYME Q10(Ubiquinone)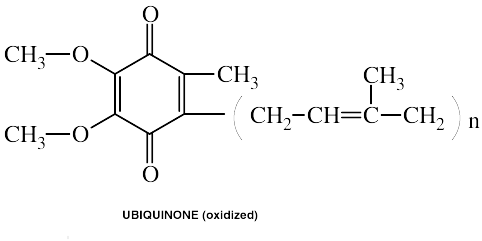
0.0 Overview

0.0 Overview
1) Class
a) This drug is a member of the following class(es):
Coenzyme
Nutriceutical
Nutritive Agent
Vitamin Combination, Adult Formula
Nutriceutical
Nutritive Agent
Vitamin Combination, Adult Formula
2) Contraindications
a) Hypersensitivity to coenzyme Q10 or product components
1.0 Dosing Information
Drug Properties
Adult Dosage
1.1 Drug Properties
A) Information on specific products and dosage forms can be obtained by referring to the Tradename List (Product Index)
B) Synonyms
B) Synonyms
Coenzyme Q
Coenzyme Q10
CO Q10
Ubidecarenone
Ubiquinone 10
Coenzyme Q10
CO Q10
Ubidecarenone
Ubiquinone 10
C) Orphan Drug Status
1) Ubiquinone has been designated an orphan product for use in the treatment of mitochondrial cytopathies.
1.3 Adult Dosage
1.3.1 Normal Dosage
Intravenous route
Oral route
1.3.1.A Intravenous route
1) Intravenous coenzyme Q10 50 to 100 milligrams daily for 3 to 35 days has been given for the treatment of severe HEART FAILURE in early studies (Ishiyama et al, 1976a).
2) An intravenous dose of 1.5 milligram/kilogram once daily for 7 days was beneficial in the treatment of chronic stable ANGINA in one double-blind study (Greenberg & Frishman, 1990b).
2) An intravenous dose of 1.5 milligram/kilogram once daily for 7 days was beneficial in the treatment of chronic stable ANGINA in one double-blind study (Greenberg & Frishman, 1990b).
1.3.1.B Oral route
1) Coenzyme Q10 has commonly been given in soft gelatin capsules, with the drug dissolved in soybean oil (Mortensen, 1993a). In one study, the drug was dissolved in a sweetened emulsion of hydrogenated vegetable oils (Nutriwhip(R)) (final concentration 10 milligrams/milliliter) (Matthews et al, 1993b).
2) Coenzyme Q10 50 to 150 milligrams daily in two or three divided doses has been administered to chronic CONGESTIVE HEART FAILURE patients receiving conventional therapy (eg, digoxin, diuretics, angiotensin-converting enzyme inhibitors, calcium channel blocking agents) (Morisco et al, 1993; Lampertico & Comis, 1993b; Baggio et al, 1993a; Langsjoen et al, 1990b; Mortensen, 1993a). Most patients considered responders in these studies were given 100 milligrams daily, given either as 50 milligrams twice daily or 33.3 milligrams three times daily. Coenzyme Q10 has been continued for up to 6 years (Langsjoen et al, 1990b).
3) In patients with chronic stable ANGINA, coenzyme Q10 has been given in doses of 150 to 600 milligrams daily in divided doses (Mortensen, 1993a; Kamikawa et al, 1985b). A dose of 33.3 milligrams three times daily was effective in the treatment of HYPERTENSION in one controlled study involving patients with low serum levels of the coenzyme Q10 and low succinate dehydrogenase coenzyme Q10 reductase activity (Greenberg & Frishman, 1990b).
4) Doses of 100 milligrams daily for 14 days prior to surgery, followed by postsurgical administration of 100 milligrams daily for 30 days, have been used for myocardial preservation in high-risk patients undergoing CARDIAC SURGERY (Judy et al, 1993a). Other investigators have employed doses of 150 to 200 milligrams daily for five to seven days prior to surgery (Chen et al, 1994).
5) A dose of 25 milligrams orally twice daily has been given for the treatment of PERIODONTAL DISEASE (Wilkinson et al, 1976a).
2) Coenzyme Q10 50 to 150 milligrams daily in two or three divided doses has been administered to chronic CONGESTIVE HEART FAILURE patients receiving conventional therapy (eg, digoxin, diuretics, angiotensin-converting enzyme inhibitors, calcium channel blocking agents) (Morisco et al, 1993; Lampertico & Comis, 1993b; Baggio et al, 1993a; Langsjoen et al, 1990b; Mortensen, 1993a). Most patients considered responders in these studies were given 100 milligrams daily, given either as 50 milligrams twice daily or 33.3 milligrams three times daily. Coenzyme Q10 has been continued for up to 6 years (Langsjoen et al, 1990b).
3) In patients with chronic stable ANGINA, coenzyme Q10 has been given in doses of 150 to 600 milligrams daily in divided doses (Mortensen, 1993a; Kamikawa et al, 1985b). A dose of 33.3 milligrams three times daily was effective in the treatment of HYPERTENSION in one controlled study involving patients with low serum levels of the coenzyme Q10 and low succinate dehydrogenase coenzyme Q10 reductase activity (Greenberg & Frishman, 1990b).
4) Doses of 100 milligrams daily for 14 days prior to surgery, followed by postsurgical administration of 100 milligrams daily for 30 days, have been used for myocardial preservation in high-risk patients undergoing CARDIAC SURGERY (Judy et al, 1993a). Other investigators have employed doses of 150 to 200 milligrams daily for five to seven days prior to surgery (Chen et al, 1994).
5) A dose of 25 milligrams orally twice daily has been given for the treatment of PERIODONTAL DISEASE (Wilkinson et al, 1976a).
2.0 Pharmacokinetics
Onset and Duration
Drug Concentration Levels
ADME
2.1 Onset and Duration
A) Onset
1) Peak Response
a) Congestive heart failure, oral: 2 weeks to 3 months (Lampertico & Comis, 1993; Greenberg & Frishman, 1990a; Langsjoen et al, 1988).
1) Significant clinical and hemodynamic improvement in patients with congestive heart failure has been reported 2 weeks to 3 months after initiation of coenzyme Q10 therapy (average, one month) (Lampertico & Comis, 1993; Greenberg & Frishman, 1990a; Langsjoen et al, 1988). Blood levels of coenzyme Q10 increased significantly after initiation of therapy in these patients (Langsjoen et al, 1990a; Langsjoen et al, 1988).
1) Significant clinical and hemodynamic improvement in patients with congestive heart failure has been reported 2 weeks to 3 months after initiation of coenzyme Q10 therapy (average, one month) (Lampertico & Comis, 1993; Greenberg & Frishman, 1990a; Langsjoen et al, 1988). Blood levels of coenzyme Q10 increased significantly after initiation of therapy in these patients (Langsjoen et al, 1990a; Langsjoen et al, 1988).
2.2 Drug Concentration Levels
A) Therapeutic Drug Concentration
1) Angina, 2.2 mcg/mL (Kamikawa et al, 1985a).
a) Normal blood levels of coenzyme Q10 have ranged from 0.7 to 1 mcg/mL in various studies (Lockwood et al, 1994; Triolo et al, 1994; Nishikawa et al, 1989).
b) In patients with chronic stable angina, increases in blood coenzyme Q10 levels (from 0.9 to 2.2 mcg/mL) were correlated with increases in exercise duration during four weeks of therapy with doses of 150 mg daily (Kamikawa et al, 1985a).
2) Cardiac surgery, not established (Judy et al, 1993).
a) Poor preservation of myocardial function, with a delayed and complicated recovery period, has been associated with coenzyme Q10 blood levels of less than 0.5 mcg/mL in high-risk cardiac surgery patients (Judy et al, 1993).
b) Normal blood levels of coenzyme Q10 have ranged from 0.7 to 1 mcg/mL in various studies (Lockwood et al, 1994; Triolo et al, 1994; Nishikawa et al, 1989).
3) Congestive heart failure, 2 to 2.5 mcg/mL or more (Langsjoen et al, 1990a; Langsjoen et al, 1988).
a) Normal blood levels of coenzyme Q10 have ranged from 0.7 to 1 mcg/mL in various studies (Lockwood et al, 1994; Triolo et al, 1994; Nishikawa et al, 1989).
a) Normal blood levels of coenzyme Q10 have ranged from 0.7 to 1 mcg/mL in various studies (Lockwood et al, 1994; Triolo et al, 1994; Nishikawa et al, 1989).
b) In patients with chronic stable angina, increases in blood coenzyme Q10 levels (from 0.9 to 2.2 mcg/mL) were correlated with increases in exercise duration during four weeks of therapy with doses of 150 mg daily (Kamikawa et al, 1985a).
2) Cardiac surgery, not established (Judy et al, 1993).
a) Poor preservation of myocardial function, with a delayed and complicated recovery period, has been associated with coenzyme Q10 blood levels of less than 0.5 mcg/mL in high-risk cardiac surgery patients (Judy et al, 1993).
b) Normal blood levels of coenzyme Q10 have ranged from 0.7 to 1 mcg/mL in various studies (Lockwood et al, 1994; Triolo et al, 1994; Nishikawa et al, 1989).
3) Congestive heart failure, 2 to 2.5 mcg/mL or more (Langsjoen et al, 1990a; Langsjoen et al, 1988).
a) Normal blood levels of coenzyme Q10 have ranged from 0.7 to 1 mcg/mL in various studies (Lockwood et al, 1994; Triolo et al, 1994; Nishikawa et al, 1989).
B) Time to Peak Concentration
1) Oral: 5 to 10 hours (Greenberg & Frishman, 1990a).
a) Following oral doses of coenzyme Q10 100 mg in healthy subjects, a mean peak blood level of 1 mcg/mL has been reported between 5 and 10 hours (mean, 6.5 hours). With administration of 100 mg three times daily, mean steady-state levels were 5.4 mcg/mL; 90% of this level was achieved after 4 days (Greenberg & Frishman, 1990a).
b) In high-risk cardiac surgery patients, administration of coenzyme Q10 100 mg daily for two weeks prior to surgery increased blood coenzyme Q10 levels from 0.6 mcg/mL (before treatment) to 1.4 mcg/mL at the time of surgery; levels of 1.3 mcg/mL and 1 mcg/mL were reported after cardiac cooling and after cardiac rewarming and reperfusion, respectively. Blood levels were maintained above 1 mcg/mL with continued therapy after surgery (100 mg daily for 30 days). A myocardial preserving effect of coenzyme Q10 was reported with this dose schedule (Judy et al, 1993).
c) Blood levels of coenzyme Q10 increased 3-fold (from 1.4 to 4.5 mcg/mL) after 2 months of treatment with doses of 300 mg daily in patients with mitochondrial disease (Matthews et al, 1993a).
d) In patients with congestive heart failure, blood levels of coenzyme Q10 have increased from 0.8 to 2 mcg/mL after three months of therapy with doses of 100 mg daily; levels remained stable with continued administration (Langsjoen et al, 1990a). A similar rise (from 0.9 to 2.2 mcg/mL) has been observed with a dose of 150 mg daily for one month in chronic stable angina patients (Kamikawa et al, 1985a).
e) A method for determining coenzyme Q10 in blood, with 100% recovery, has been described (Vadhanavikit et al, 1984).
a) Following oral doses of coenzyme Q10 100 mg in healthy subjects, a mean peak blood level of 1 mcg/mL has been reported between 5 and 10 hours (mean, 6.5 hours). With administration of 100 mg three times daily, mean steady-state levels were 5.4 mcg/mL; 90% of this level was achieved after 4 days (Greenberg & Frishman, 1990a).
b) In high-risk cardiac surgery patients, administration of coenzyme Q10 100 mg daily for two weeks prior to surgery increased blood coenzyme Q10 levels from 0.6 mcg/mL (before treatment) to 1.4 mcg/mL at the time of surgery; levels of 1.3 mcg/mL and 1 mcg/mL were reported after cardiac cooling and after cardiac rewarming and reperfusion, respectively. Blood levels were maintained above 1 mcg/mL with continued therapy after surgery (100 mg daily for 30 days). A myocardial preserving effect of coenzyme Q10 was reported with this dose schedule (Judy et al, 1993).
c) Blood levels of coenzyme Q10 increased 3-fold (from 1.4 to 4.5 mcg/mL) after 2 months of treatment with doses of 300 mg daily in patients with mitochondrial disease (Matthews et al, 1993a).
d) In patients with congestive heart failure, blood levels of coenzyme Q10 have increased from 0.8 to 2 mcg/mL after three months of therapy with doses of 100 mg daily; levels remained stable with continued administration (Langsjoen et al, 1990a). A similar rise (from 0.9 to 2.2 mcg/mL) has been observed with a dose of 150 mg daily for one month in chronic stable angina patients (Kamikawa et al, 1985a).
e) A method for determining coenzyme Q10 in blood, with 100% recovery, has been described (Vadhanavikit et al, 1984).
2.3 ADME
Absorption
Distribution
Metabolism
Excretion
Elimination Half-life
2.3.1 Absorption
A) Bioavailability
1) Oral: absorbed slowly (Greenberg & Frishman, 1990a).
a) In a solubilized dosage form, coenzyme Q10 is absorbed significantly more efficiently than from conventionally marketed oral dosage forms such as softgel capsules containing a coenzyme Q10 suspension in oil, powder-filled hardshell capsules, or powder-based tablets (Chopra et al, 1998). Typically, coenzyme Q10 is absorbed slowly from the gastrointestinal tract, due to the high molecular weight of coenzyme Q10 and its low water solubility (Greenberg & Frishman, 1990a).
a) In a solubilized dosage form, coenzyme Q10 is absorbed significantly more efficiently than from conventionally marketed oral dosage forms such as softgel capsules containing a coenzyme Q10 suspension in oil, powder-filled hardshell capsules, or powder-based tablets (Chopra et al, 1998). Typically, coenzyme Q10 is absorbed slowly from the gastrointestinal tract, due to the high molecular weight of coenzyme Q10 and its low water solubility (Greenberg & Frishman, 1990a).
2.3.2 Distribution
A) Distribution Sites
1) OTHER DISTRIBUTION SITES
a) LIVER, amount unknown (Greenberg & Frishman, 1990a).
1) After oral absorption or intravenous administration, coenzyme Q10 is taken up by chylomicrons. Most of an exogenous dose is distributed to the liver and incorporated into very-low-density lipoprotein (Greenberg & Frishman, 1990a).
2) Endogenous coenzyme Q10 is found in relatively high concentrations in the heart, liver, kidney, and pancreas. Intracellularly, most of the coenzyme (40% to 50%) is found in the mitochondria (inner mitochondrial membrane). Other intracellular distribution sites are: cytosolic 5% to 10%, microsomal 15% to 20%, and nucleus 25% to 30% (Greenberg & Frishman, 1990a).
b) SEMINAL FLUID, 127.1 +/- 1.9 ng/mL (Balercia et al, 2004).
1) In an open, uncontrolled trial, 22 men (mean age, 31 years) received coenzyme Q10 100 milligrams twice daily orally for 6 months. After 6 months of treatment, coenzyme Q10 levels increased in seminal plasma, with the mean value rising from 42 +/- 5.1 nanograms/milliliter at baseline to 127.1 +/- 1.9 ng/mL (p less than .005) (Balercia et al, 2004).
c) SPERM, 6.5 +/- 0.3 ng/10(6) cells (Balercia et al, 2004).
1) In an open, uncontrolled trial, 22 men (mean age, 31 years) received coenzyme Q10 100 milligrams twice daily orally for 6 months. Sperm cells demonstrated a significant increase in coenzyme Q10 content (from 3.1 +/- 0.4 to 6.5 +/- 0.3 ng/10(6) cells, p less than 0.5) (Balercia et al, 2004).
d) TISSUES, amount unknown (Greenberg & Frishman, 1990a).
1) After incorporation into very-low-density lipoprotein in the liver, the coenzyme is subsequently concentrated in various tissues, including the adrenals, spleen, kidney, lung, and myocardium (Greenberg & Frishman, 1990a).
2) Endogenous coenzyme Q10 is found in relatively high concentrations in the heart, liver, kidney, and pancreas. Intracellularly, most of the coenzyme (40% to 50%) is found in the mitochondria (inner mitochondrial membrane). Other intracellular distribution sites are: cytosolic 5% to 10%, microsomal 15% to 20%, and nucleus 25% to 30% (Greenberg & Frishman, 1990a).
1) After oral absorption or intravenous administration, coenzyme Q10 is taken up by chylomicrons. Most of an exogenous dose is distributed to the liver and incorporated into very-low-density lipoprotein (Greenberg & Frishman, 1990a).
2) Endogenous coenzyme Q10 is found in relatively high concentrations in the heart, liver, kidney, and pancreas. Intracellularly, most of the coenzyme (40% to 50%) is found in the mitochondria (inner mitochondrial membrane). Other intracellular distribution sites are: cytosolic 5% to 10%, microsomal 15% to 20%, and nucleus 25% to 30% (Greenberg & Frishman, 1990a).
b) SEMINAL FLUID, 127.1 +/- 1.9 ng/mL (Balercia et al, 2004).
1) In an open, uncontrolled trial, 22 men (mean age, 31 years) received coenzyme Q10 100 milligrams twice daily orally for 6 months. After 6 months of treatment, coenzyme Q10 levels increased in seminal plasma, with the mean value rising from 42 +/- 5.1 nanograms/milliliter at baseline to 127.1 +/- 1.9 ng/mL (p less than .005) (Balercia et al, 2004).
c) SPERM, 6.5 +/- 0.3 ng/10(6) cells (Balercia et al, 2004).
1) In an open, uncontrolled trial, 22 men (mean age, 31 years) received coenzyme Q10 100 milligrams twice daily orally for 6 months. Sperm cells demonstrated a significant increase in coenzyme Q10 content (from 3.1 +/- 0.4 to 6.5 +/- 0.3 ng/10(6) cells, p less than 0.5) (Balercia et al, 2004).
d) TISSUES, amount unknown (Greenberg & Frishman, 1990a).
1) After incorporation into very-low-density lipoprotein in the liver, the coenzyme is subsequently concentrated in various tissues, including the adrenals, spleen, kidney, lung, and myocardium (Greenberg & Frishman, 1990a).
2) Endogenous coenzyme Q10 is found in relatively high concentrations in the heart, liver, kidney, and pancreas. Intracellularly, most of the coenzyme (40% to 50%) is found in the mitochondria (inner mitochondrial membrane). Other intracellular distribution sites are: cytosolic 5% to 10%, microsomal 15% to 20%, and nucleus 25% to 30% (Greenberg & Frishman, 1990a).
2.3.3 Metabolism
A) Metabolism Sites and Kinetics
1) LIVER, extent unknown (Greenberg & Frishman, 1990a).
a) The metabolic fate of coenzyme Q10 is unclear. The majority of an absorbed dose is distributed to the liver and incorporated into very-low-density lipoprotein (Greenberg & Frishman, 1990a).
a) The metabolic fate of coenzyme Q10 is unclear. The majority of an absorbed dose is distributed to the liver and incorporated into very-low-density lipoprotein (Greenberg & Frishman, 1990a).
2.3.4 Excretion
A) Total Body Clearance
1) minimal (Greenberg & Frishman, 1990a).
a) Coenzyme Q10 appears to have a low plasma clearance (Greenberg & Frishman, 1990a).
a) Coenzyme Q10 appears to have a low plasma clearance (Greenberg & Frishman, 1990a).
B) Other
1) OTHER EXCRETION
a) BILE, extent unknown (Greenberg & Frishman, 1990a).
1) Coenzyme Q10 is predominantly excreted via the biliary tract (Greenberg & Frishman, 1990a).
b) FECES, 60% (Greenberg & Frishman, 1990a).
1) Over 60% of oral doses are recovered in the feces during chronic administration (Greenberg & Frishman, 1990a).
1) Coenzyme Q10 is predominantly excreted via the biliary tract (Greenberg & Frishman, 1990a).
b) FECES, 60% (Greenberg & Frishman, 1990a).
1) Over 60% of oral doses are recovered in the feces during chronic administration (Greenberg & Frishman, 1990a).
2.3.5 Elimination Half-life
A) Parent Compound
1) ELIMINATION HALF-LIFE
a) 34 hours (Greenberg & Frishman, 1990a).
3.0 Cautions
Contraindications
Precautions
Adverse Reactions
Drug Interactions
3.1 Contraindications
A) Hypersensitivity to coenzyme Q10 or product components
3.2 Precautions
A) Biliary obstruction (potential accumulation of coenzyme Q10)
B) Concomitant therapy with hypolipidemic agents (lower plasma concentrations of endogenous coenzyme Q10 in hyperlipidemic patients) (Watts et al, 1993)
C) Concomitant therapy with oral hypoglycemic agents (potentially inhibit effects of exogenous administration) (Greenberg & Frishman, 1990)
D) Diabetes mellitus (possible reduced insulin requirements) (Matthews et al, 1993)
E) Hepatic insufficiency (potential accumulation of coenzyme Q10)
F) Renal insufficiency (pharmacokinetic data unavailable)
B) Concomitant therapy with hypolipidemic agents (lower plasma concentrations of endogenous coenzyme Q10 in hyperlipidemic patients) (Watts et al, 1993)
C) Concomitant therapy with oral hypoglycemic agents (potentially inhibit effects of exogenous administration) (Greenberg & Frishman, 1990)
D) Diabetes mellitus (possible reduced insulin requirements) (Matthews et al, 1993)
E) Hepatic insufficiency (potential accumulation of coenzyme Q10)
F) Renal insufficiency (pharmacokinetic data unavailable)
3.3 Adverse Reactions
Dermatologic Effects
Gastrointestinal Effects
Hematologic Effects
Hepatic Effects
Neurologic Effects
Ophthalmic Effects
Other
3.3.2 Dermatologic Effects
3.3.2.A Dermatological finding
1) SKIN RASH and PRURITUS are infrequent complications of oral coenzyme Q10 therapy (less than 0.5% of patients) (Lampertico & Comis, 1993a; Langsjoen et al, 1990; Baggio et al, 1993). Oral coenzyme Q10 was discontinued due to the appearance of a rash in one patient in one study (Baggio et al, 1993).
2) An itching EXANTHEMA was described in 2 of 21 heart failure patients treated with intravenous coenzyme Q10 (50 to 100 mg) (Ishiyama et al, 1976).
2) An itching EXANTHEMA was described in 2 of 21 heart failure patients treated with intravenous coenzyme Q10 (50 to 100 mg) (Ishiyama et al, 1976).
3.3.4 Gastrointestinal Effects
3.3.4.A Gastrointestinal tract finding
1) The most common adverse effects of oral coenzyme Q10 are gastrointestinal in nature, and include NAUSEA, EPIGASTRIC DISCOMFORT, DIARRHEA, HEARTBURN, and APPETITE SUPPRESSION (Rengo et al, 1993a; Baggio et al, 1993; Lampertico & Comis, 1993a; Greenberg & Frishman, 1990; Matthews et al, 1993; Kamikawa et al, 1985). However, their incidence has been less than 1% in large studies (Greenberg & Frishman, 1990; Lampertico & Comis, 1993a; Baggio et al, 1993).
2) Gastrointestinal effects of oral coenzyme Q10 have responded to dose reduction, and have tended to subside with continued therapy (Lampertico & Comis, 1993a; Matthews et al, 1993); withdrawal of therapy has been required only rarely (Baggio et al, 1993).
2) Gastrointestinal effects of oral coenzyme Q10 have responded to dose reduction, and have tended to subside with continued therapy (Lampertico & Comis, 1993a; Matthews et al, 1993); withdrawal of therapy has been required only rarely (Baggio et al, 1993).
3.3.5 Hematologic Effects
3.3.5.A Hematology finding
1) THROMBOCYTOPENIA was described in one of 16 patients treated with oral coenzyme Q10 for mitochondrial disease in one study (Matthews et al, 1993). However, other factors were more likely responsible (eg, viral infection, other medications). No other adverse hematologic effects have been reported in clinical trials.
3.3.6 Hepatic Effects
3.3.6.A Hepatotoxicity
1) Mild increases in serum aminotransferases have been reported occasionally with higher oral doses of coenzyme Q10 (300 mg daily) (Greenberg & Frishman, 1990). However, there are no reports of overt hepatotoxicity with the drug.
3.3.9 Neurologic Effects
3.3.9.A Central nervous system finding
1) IRRITABILITY or AGITATION, HEADACHE, and DIZZINESS have occurred rarely during oral coenzyme Q10 therapy (Baggio et al, 1993; Wilkinson et al, 1976; Lampertico & Comis, 1993a). It is unclear if any of these effects are definitely drug-related.
3.3.10 Ophthalmic Effects
3.3.10.A Eye / vision finding
1) PHOTOPHOBIA has been reported rarely during oral therapy with coenzyme Q10 (Baggio et al, 1993), although a causal relationship is doubtful.
3.3.16 Other
3.3.16.A Influenza-like symptoms
1) Influenza-like symptoms were described in one of 10 patients receiving oral coenzyme Q10 for periodontal disease in one study, and treatment was discontinued (Wilkinson et al, 1976). However, a causal relationship to coenzyme Q10 is doubtful.
3.5 Drug Interactions
3.5.1 Drug-Drug Combinations
Acenocoumarol
Ancrod
Anisindione
Antithrombin III Human
Bivalirudin
Danaparoid
Defibrotide
Dermatan Sulfate
Desirudin
Dicumarol
Fondaparinux
Heparin
Pentosan Polysulfate Sodium
Phenindione
Phenprocoumon
Warfarin
3.5.1.A Acenocoumarol
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.B Ancrod
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.C Anisindione
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.D Antithrombin III Human
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.E Bivalirudin
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.F Danaparoid
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.G Defibrotide
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.H Dermatan Sulfate
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
3.5.1.I Desirudin
1) Interaction Effect: reduced anticoagulant effectiveness
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
2) Summary: Coenzyme Q10 had no effect on the international normalized ratio (INR) in patients stable on warfarin in a randomized, double-blind, placebo-controlled, crossover trial (Engelsen et al, 2003a). Case reports associate coenzyme Q10 therapy with decreased INR in patients taking warfarin (Spigset, 1994a). Caution is advised if patients take coenzyme Q10 and warfarin.
3) Severity: moderate
4) Onset: delayed
5) Substantiation: probable
6) Clinical Management: Caution is advised if coenzyme Q10 and warfarin are taken together. Monitor the INR to determine continued therapeutic effect.
7) Probable Mechanism: similar chemical structure of coenzyme Q10 and vitamin K2
8) Literature Reports
a) Three patients were reported to have a decrease in the international normalized ratio (INR) after addition of ubidecarenone (coenzyme Q10) to their warfarin regimens. A 68-year-old man with a stable INR of 2 to 3.5 on warfarin had an INR of 1.31 after two weeks of taking ubidecarenone 30 mg daily. Ubidecarenone was discontinued and the INR subsequently remained therapeutic. A 72-year-old man developed a pulmonary embolism after three months of taking ubidecarenone. A 70-year-old woman stable on warfarin for several years had an INR of 1.42 after two weeks of taking ubidecarenone 30 mg daily. Her INR returned to the therapeutic range after discontinuation of ubidecarenone and a temporary increase in warfarin dosage (Spigset, 1994).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
b) Coenzyme Q10 did not affect the international normalized ratio (INR) in a randomized, double-blind, placebo-controlled, crossover trial of 24 patients stable on warfarin. Patients received coenzyme Q10 100 milligrams (mg) daily in addition to their regular warfarin therapy for four weeks, then crossed over to placebo after a two-week washout. The geometric mean warfarin dose remained the same and INR was unaffected during treatment (Engelsen et al, 2003).
續下篇.....
全站熱搜





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 