Dear Healthcare Provider:
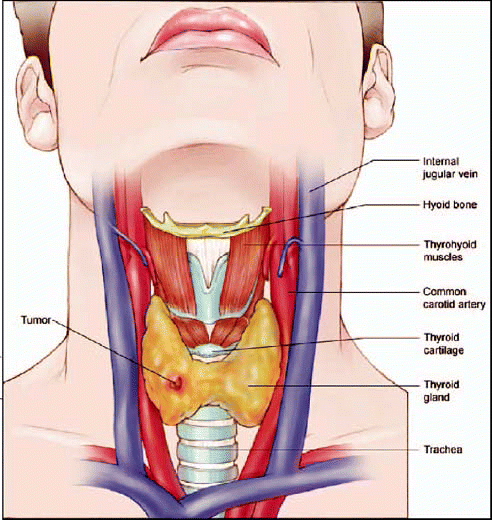
AstraZeneca Pharmaceuticals LP would like to inform you of the approval of vandetanib, a
new kinase inhibitor that has been approved by the Food and Drug Administration (FDA) for
the treatment of symptomatic or progressive medullary thyroid cancer in patients with
unresectable locally advanced or metastatic disease. Use of vandetanib in patients with
indolent, asymptomatic or slowly progressing disease should be carefully considered because
of the treatment related risks of vandetanib.
Vandetanib can prolong the QT interval and cases of Torsades de pointes and sudden
death were reported in clinical trials. Because of these risks, vandetanib is available only
through a restricted distribution program called Vandetanib REMS Program. Under the
Vandetanib REMS Program, only prescribers and pharmacies enrolled in the program
can prescribe and dispense vandetanib.
In order to prescribe vandetanib, you must:
Read this Healthcare Provider (HCP) Letter; review HCP Education Pamphlet or HCP
REMS Education Slide Set; and the vandetanib full Prescribing Information
Complete the Prescriber Training Program (online or by phone)
Complete the Prescriber Enrollment Form
To ENROLL, visit www.vandetanibrems.com or call 1-800-236-9933.
Please see the enclosed HCP Education Pamphlet that outlines the risk of QT prolongation,
Torsades de pointes and sudden death associated with vandetanib.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 