機轉圖在這邊:
圖解藥理學 20抗凝血機轉10.1
Platelets play a central role in atherothrombosis and are an important target for pharmacotherapy. In patients with acute coronary syndromes, the use of potent platelet inhibitors has been shown to reduce the rate of thrombotic events at the cost of increased bleeding.1-3 In contrast, among patients with stable atherosclerosis, a reduced rate of thrombotic events with antiplatelet therapy in addition to aspirin therapy has not been established.4
Thrombin is a serine protease that is critical in thrombosis. In addition to generating fibrin, thrombin is a potent agonist of platelets through interaction with protease-activated receptors (PARs).5Vorapaxar (SCH 530348, Merck) is a competitive and selective antagonist of PAR-1, the major thrombin receptor on human platelets. Vorapaxar potently inhibits thrombin-induced platelet aggregation.6,7 This study, called the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2P)–Thrombolysis in Myocardial Infarction (TIMI) 50 trial, was designed to evaluate the efficacy and safety of vorapaxar in reducing atherothrombotic events in patients with established atherosclerosis who were receiving standard therapy. In addition to investigating vorapaxar specifically, the trial more broadly tested the hypothesis that the intensification of antiplatelet therapy by adding an agent with a different pharmacologic target is beneficial for secondary prevention in patients with stable disease and a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
METHODS
Study Design and Oversight
The TRA 2P–TIMI 50 trial was a multinational, double-blind, placebo-controlled trial8 that was conducted at 1032 sites in 32 countries (see the Supplementary Appendix, available with the full text of this article at NEJM.org). The trial was sponsored by Merck and was designed by the TIMI Study Group in conjunction with the steering committee and trial sponsor. The protocol was approved by the relevant ethics committees at all participating centers. The raw database was provided to the TIMI Study Group, which carried out the data analyses independently of the sponsor, prepared this report, and made the decision to submit the manuscript for publication. The members of the TIMI Study Group assume responsibility for the accuracy and completeness of the data and all analyses and for the fidelity of this report to the study protocol, which is available at NEJM.org.
Study Population
Eligible patients had a history of atherosclerosis, which was defined as a spontaneous myocardial infarction or ischemic stroke within the previous 2 weeks to 12 months or peripheral arterial disease associated with a history of intermittent claudication in conjunction with either an ankle–brachial index of less than 0.85 or previous revascularization for limb ischemia. By design, enrollment of patients with a qualifying diagnosis of either stroke or peripheral arterial disease was to end when the number enrolled reached approximately 15% of the total anticipated sample size.
Patients were ineligible if they were planning to undergo a revascularization procedure, had a history of bleeding diathesis, had recent active abnormal bleeding, were receiving ongoing treatment with warfarin, or had active hepatobiliary disease. The full eligibility criteria have been reported previously.8 Written informed consent was obtained from all patients.
Randomization and Study Treatment
Eligible patients were randomly assigned in a 1:1 ratio to receive either vorapaxar (2.5 mg daily) or matched placebo by a central computerized system with hierarchical stratification according to the qualifying diagnosis (myocardial infarction, stroke, or peripheral arterial disease) and the responsible physician's intent to administer a thienopyridine (see the Methods section in the Supplementary Appendix). Vorapaxar and placebo were administered orally in a blinded fashion once daily until the end of follow-up. Study therapy was to be interrupted if the patient required treatment with either a potent inhibitor of the cytochrome P-450 3A4 (CYP3A4) enzyme system or warfarin in conjunction with a thienopyridine. All concomitant medical therapy, including the use of other antiplatelet agents, was managed by the clinicians at the study sites who were responsible for the care of the patients, according to local standards of care.
In January 2011, after completion of enrollment and a median of 24 months of follow-up, the data and safety monitoring board reported an excess of intracranial hemorrhage in patients with a history of stroke in the vorapaxar group and recommended discontinuation of the drug in all patients with previous stroke, including those with a new stroke during the trial. The board also recommended continuation of the trial in patients without a history of stroke. The protocol was amended accordingly (see the Methods section in the Supplementary Appendix).
End Points
The protocol-defined primary efficacy end point was a composite of cardiovascular death, myocardial infarction, stroke, or recurrent ischemia leading to urgent coronary revascularization. The major secondary end point was a composite of cardiovascular death, myocardial infarction, or stroke. Before the database was locked and during blinded treatment, the investigators reviewed data from the newly completed Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial (ClinicalTrials.gov number, NCT00527943).9 On the basis of data from that trial, the steering committee amended the main data-analysis plan to reorder the hierarchy of efficacy analyses, defining as the primary end point the composite of cardiovascular death, myocardial infarction, or stroke. The composite of cardiovascular death, myocardial infarction, stroke, or urgent coronary revascularization became the major secondary end point. Definitions of the components of these composite end points are provided in the Supplementary Appendix.
We assessed bleeding using the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) classification system and the TIMI classification system, with GUSTO moderate or severe bleeding defined as the safety end point of primary interest. A clinical-events committee whose members were unaware of the study-group assignments adjudicated all components of the primary and major secondary efficacy end points and bleeding episodes.
Statistical Analysis
We calculated that 2279 events would be required to provide a power of at least 90% to detect a 15% relative risk reduction in the composite end point of cardiovascular death, myocardial infarction, stroke, or urgent coronary revascularization in the vorapaxar group, as compared with the placebo group. We estimated that 1400 events would be required to provide a power of at least 85% to detect the same relative treatment effect with respect to the composite end point of cardiovascular death, myocardial infarction, or stroke.
The primary efficacy analysis was conducted on an intention-to-treat basis among all patients who underwent randomization. After the data and safety monitoring board recommended discontinuation of vorapaxar in all patients with previous stroke, the protocol was amended to include supplementary evaluation of efficacy in patients who qualified for the trial with a diagnosis of myocardial infarction or peripheral arterial disease without a history of stroke before randomization. In addition, an analysis of efficacy that was restricted to patients with the qualifying diagnosis of myocardial infarction alone was specified in the original analysis plan before the board's recommendation.
The efficacy analyses were performed with the use of a Cox proportional-hazards model, with the study group and stratification factors at randomization as covariates. Cumulative event rates were calculated with the use of the Kaplan–Meier method at 3 years. Safety analyses were performed among patients who received one or more doses of a study drug and included events through 60 days after premature cessation of study therapy or 30 days after a final visit at the conclusion of the trial. On the basis of one planned interim analysis of efficacy, a P value of less than 0.049 was considered to indicate statistical significance for the final analysis,8 and a P value of less than 0.05 was deemed to indicate a significant interaction. All reported P values are two-sided.
RESULTS
Study Patients and Follow-up
From September 26, 2007, through November 13, 2009, a total of 26,449 patients were enrolled in the trial. Of these, 13,225 were randomly assigned to receive vorapaxar, and 13,244 to receive placebo (Figure S1 in the Supplementary Appendix).
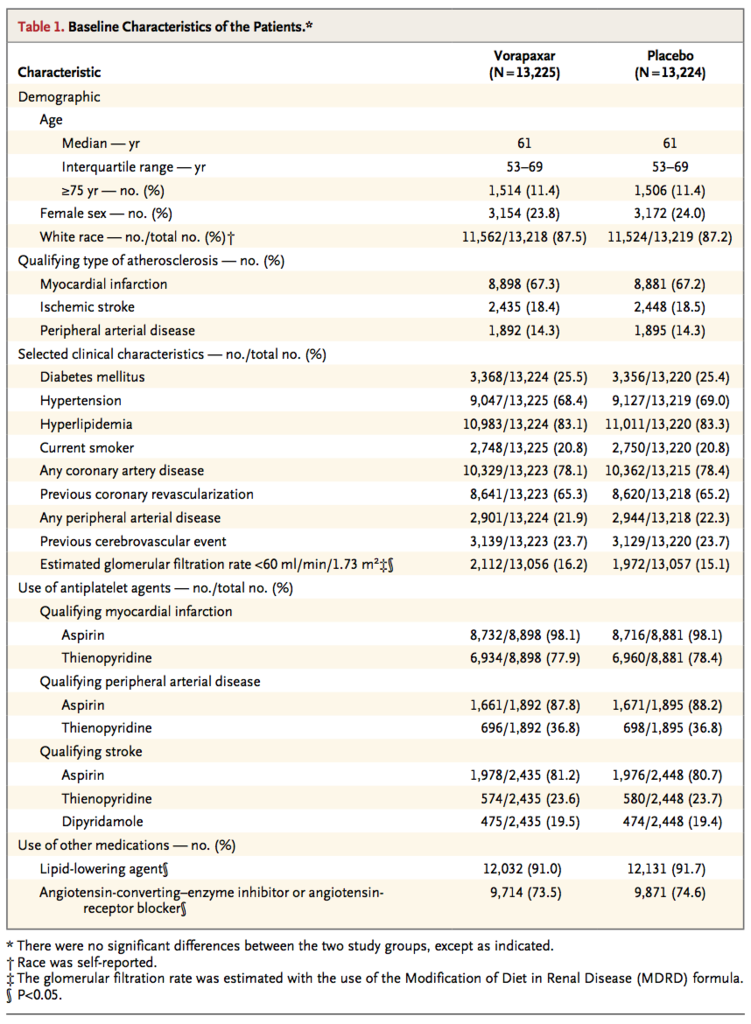
Baseline characteristics of the patients are shown in Table 1
The qualifying diagnosis for enrollment was myocardial infarction in two thirds of the patients, stroke in 18%, and peripheral arterial disease in 14%. A total of 94% of the patients were treated with aspirin. At baseline, a thienopyridine was being administered in a majority of patients with a qualifying diagnosis of myocardial infarction but in only a minority of patients with a qualifying diagnosis of stroke or peripheral arterial disease. Only 177 patients (0.7%) received prasugrel during the study.
The longest duration of follow-up was 49 months, with a median follow-up of 30 months (interquartile range, 24 to 36). Details of follow-up and loss to follow-up are provided in the Supplementary Appendix. The last date of patient contact was December 23, 2011, and the trial database was locked on January 9, 2012.
Efficacy End Points
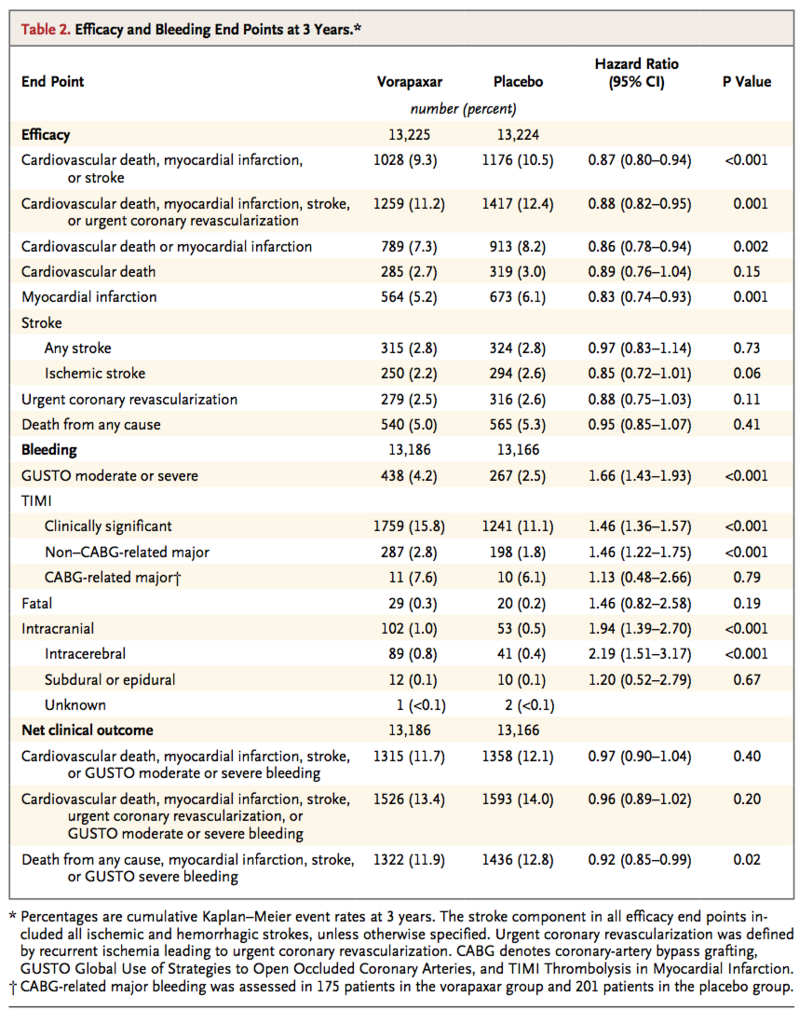
At 3 years, the primary end point of cardiovascular death, myocardial infarction, or stroke had occurred in 1028 patients (9.3%) in the vorapaxar group, as compared with 1176 patients (10.5%) in the placebo group (hazard ratio, 0.87; 95% confidence interval [CI], 0.80 to 0.94; P<0.001) (Table 2
and Figure 1A
The major secondary end point of cardiovascular death, myocardial infarction, stroke, or urgent coronary revascularization occurred in 1259 patients (11.2%) in the vorapaxar group, as compared with 1417 patients (12.4%) in the placebo group (hazard ratio, 0.88; 95% CI, 0.82 to 0.95; P=0.001) (Table 2 and Figure 1B). The rate of cardiovascular death or myocardial infarction was reduced from 8.2% among patients in the placebo group to 7.3% among patients in the vorapaxar group (P=0.002) (Table 2, and Figure S2 in the Supplementary Appendix). Individual components of these composite end points are also shown in Table 2. The rate of death from any cause did not differ significantly between the vorapaxar group and the placebo group (5.0% and 5.3%, respectively; hazard ratio, 0.95; 95% CI, 0.85 to 1.07; P=0.41). (Additional prespecified efficacy end points are shown in Tables S1 and S2 in the Supplementary Appendix.)
Among patients with no history of stroke, the primary end point occurred in 8.3% of patients in the vorapaxar group, as compared with 9.6% of those in the placebo group (hazard ratio, 0.84; 95% CI, 0.76 to 0.93; P<0.001) (Table S3 in the Supplementary Appendix). There was no significant heterogeneity for the benefit of vorapaxar on the rate of cardiovascular death, myocardial infarction, or stroke across most of the major subgroups examined, including those defined according to the use or nonuse of a thienopyridine (Figure S3 in the Supplementary Appendix). However, among patients who weighed less than 60 kg, the use of vorapaxar did not have a favorable influence on this outcome (P=0.03 for interaction). Additional analyses of efficacy in subgroups that were defined according to the qualifying diagnosis of atherosclerosis (myocardial infarction, stroke, or peripheral arterial disease) and the presence or absence of a history of stroke are provided in Table S3 and Figure S3 in the Supplementary Appendix.
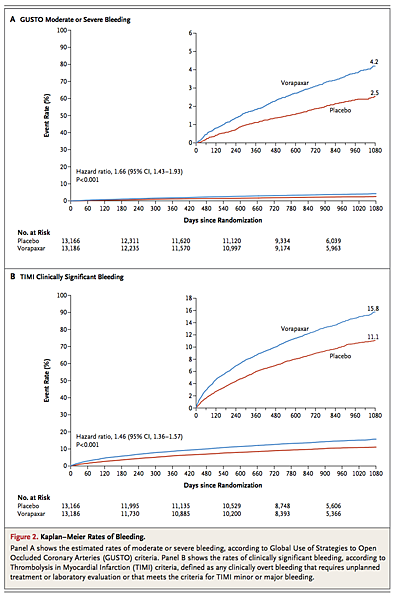
There was no evidence of heterogeneity in the effect of vorapaxar on moderate or severe bleeding in major subgroups (Figure S4 in the Supplementary Appendix). Rates of TIMI clinically significant bleeding (Figure 2B) and rates of TIMI major bleeding not related to coronary-artery bypass grafting were also both significantly increased in the vorapaxar group as compared with the placebo group (P<0.001) (Table 2).
Overall, intracranial hemorrhage occurred in 102 patients (1.0%) in the vorapaxar group, as compared with 53 patients (0.5%) in the placebo group (hazard ratio, 1.94; 95% CI, 1.39 to 2.70; P<0.001) (Table 2, and Figure S5 in the Supplementary Appendix). Fatal bleeding occurred in 29 patients (0.3%) in the vorapaxar group, as compared with 20 patients (0.2%) in the placebo group (hazard ratio, 1.46; 95% CI, 0.82 to 2.58; P=0.19). Among patients with a history of stroke, the rate of intracranial hemorrhage in the vorapaxar group was 2.4%, as compared with 0.9% in the placebo group (P<0.001), with corresponding rates of fatal bleeding of 0.5% and 0.3% (P=0.46) (Table S3 in the Supplementary Appendix). Among patients without a history of stroke, the rates of intracranial hemorrhage were lower in the two study groups (0.6% in the vorapaxar group and 0.4% in the placebo group, P=0.049), as were the rates of fatal bleeding (0.3% and 0.2%, respectively; P=0.30). Additional safety analyses are shown in Tables S3 and S4 in the Supplementary Appendix.
Net Clinical Outcome
A composite end point termed net clinical outcome, comprising the primary efficacy and safety end points, was prespecified (Table 2). The composite of cardiovascular death, myocardial infarction, stroke, or GUSTO moderate or severe bleeding occurred in 1315 patients (11.7%) in the vorapaxar group and in 1358 patients (12.1%) in the placebo group (hazard ratio, 0.97; 95% CI, 0.90 to 1.04; P=0.40). Other net clinical outcomes in all patients and in patients without stroke are reported inTable 2, and Table S4 in the Supplementary Appendix.
DISCUSSION
In previous studies, it has not been established whether the addition of another antiplatelet agent to aspirin therapy would reduce the rate of thrombotic events in patients with stable atherosclerotic disease.4 In our large randomized trial, the PAR-1 antagonist vorapaxar significantly reduced the rate of cardiovascular death, myocardial infarction, or stroke in patients with a history of atherothrombosis who were receiving standard therapy. However, the reduction in cardiovascular events came at the cost of increased bleeding. This benefit and risk emerged early and continued to accrue throughout follow-up. Our findings show that inhibition of another platelet pathway in addition to that targeted by standard antiplatelet therapy for secondary prevention reduces the risk of recurrent thrombotic events in patients with previous atherothrombosis. This benefit was evident particularly in those whose qualifying diagnosis for participation in the trial was myocardial infarction.
The results of our study establish that interruption of the platelet-directed cellular actions of thrombin, suggested in preclinical studies to be pivotal to thrombosis,10 translates into a clinical effect on major thrombotic events. In our study, antagonism of PAR-1 was complementary to inhibition of the thromboxane A2 and P2Y12-receptor pathways with aspirin and thienopyridines, with respect to the protective effect against recurrent thrombosis. We did not find evidence of significant heterogeneity of the effect of vorapaxar on the basis of treatment with clopidogrel. When vorapaxar was studied in the TRACER trial for the management of acute coronary syndromes, there was a nonsignificant trend toward a reduction in the rate of cardiovascular death, myocardial infarction, stroke, recurrent ischemia with hospitalization, or urgent coronary revascularization and an exploratory finding of a reduction in the rate of cardiovascular death, myocardial infarction, or stroke, along with a significant increase in the risk of intracranial hemorrhage.9 Previous smaller phase 2 trials of vorapaxar11,12 and of atopaxar,13,14 another PAR-1 antagonist, have also revealed trends toward reductions in recurrent thrombotic events but without evidence of an increased risk of intracranial hemorrhage.
During the trial, the data and safety monitoring board recommended the discontinuation of vorapaxar in patients with a history of stroke on the basis of an excess of intracranial hemorrhage in such patients. We therefore carried out a separate analysis that was confined to patients without a history of stroke and found a significant benefit in this subgroup. In addition, in a prespecified analysis involving patients with a qualifying diagnosis of myocardial infarction, vorapaxar reduced the relative risk of the primary end point by 20%. In the largest previous placebo-controlled trial of antiplatelet therapy for secondary prevention in patients with stable atherothrombosis or at high risk for vascular disease, clopidogrel plus aspirin was no better than aspirin alone in the overall cohort.4Nevertheless, in an exploratory analysis involving 3846 patients with previous myocardial infarction in that trial, the addition of clopidogrel reduced the risk of cardiovascular death, myocardial infarction, or stroke by 23%.15 Our trial involving a substantially larger number of patients with a history of myocardial infarction shows that the addition of another antiplatelet agent to aspirin therapy over the long term can achieve further reductions in recurrent atherothrombotic events in this population.
The reduction in thrombotic events with vorapaxar came with significant increases in bleeding. Although our findings show that additional reductions in atherothrombosis can be achieved, this benefit must be weighed against the increase in bleeding risk. In the overall trial cohort, there was no significant between-group difference in the prespecified net clinical outcome (including both thrombotic end points and moderate or severe bleeding). However, weighed against the risk of severe bleeding, particularly in patients without a history of stroke, the net clinical outcome was improved in patients receiving vorapaxar.
The results of our study also add to the accumulating evidence of a heightened risk of intracranial hemorrhage among patients with a history of stroke who are treated with potent antiplatelet therapy.2,16,17 As with the use of third-generation P2Y12 inhibitors, such as prasugrel, any clinical use of vorapaxar would have to be based on an appropriate selection of patients, with the risk of bleeding balanced against that of recurrent thrombotic events. We have previously identified criteria for selecting patients who are more likely to have improved net clinical outcomes with potent antiplatelet therapy.2,18 In our study, the relative risk of bleeding, including intracranial hemorrhage, showed a similar significant increase in the vorapaxar group among patients with and those without a history of stroke. However, the absolute rate of intracranial hemorrhage (0.2% per year) among patients without a history of stroke was substantially lower than that among patients with such a history (0.8% per year). The rate of fatal bleeding was not significantly increased in the vorapaxar group.
In conclusion, the addition of vorapaxar to standard therapy reduced the risk of cardiovascular death, myocardial infarction, or stroke among patients with stable atherosclerosis, a benefit that was most apparent in patients with a history of myocardial infarction. Vorapaxar also increased the risk of moderate or severe bleeding, including intracranial hemorrhage, with the latter occurring most frequently in patients with a history of stroke.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 