Cell. 2015 Dec 17;163(7):1585-95. doi: 10.1016/j.cell.2015.11.055.
SUMMARY
Trimethylamine N-oxide (TMAO), a gut microbiota dependent metabolite, both enhances atherosclerosis in animal models and is associated with cardiovascular risks in clinical studies. Here we investigate the impact of targeted inhibition of the first step in TMAO generation, commensal microbial trimethylamine (TMA) production, on diet-induced atherosclerosis. A structural analogue of choline, 3,3-dimethyl-1-butanol (DMB), is shown to non-lethally inhibit TMA formation from cultured microbes, to inhibit distinct microbial TMA lyases, and to both inhibit TMA production from physiologic polymicrobial cultures (eg intestinal contents, human feces) and reduce TMAO levels in mice fed a high choline or carnitine diet. DMB inhibited choline diet-enhanced endogenous macrophage foam cell formation and atherosclerotic lesion development in apolipoprotein e−/− mice without alterations in circulating cholesterol levels. The present studies suggest gut microbial production of TMA specifically, and non-lethal microbial inhibitors in general, may serve as a potential therapeutic approach for the treatment of cardiometabolic diseases.
INTRODUCTION
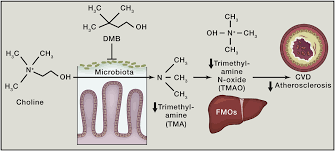
Recent studies suggest gut microbes are participants in atherosclerosis development. Specifically, choline, phosphatidylcholine and carnitine, trimethylamine (TMA)-containing nutrients abundant in foods like meat, egg yolk and high fat dairy products, serve as dietary precursors for trimethylamine N-oxide (TMAO) generation in mice and humans, a metabolite that accelerates atherosclerosis in animal models (Koeth et al., 2013; Tang et al., 2013; Wang et al., 2011). Blood TMAO levels are associated with risks for both prevalent atherosclerotic heart disease and incident major adverse cardiac events in multiple independent cohorts (Koeth et al., 2013; Lever et al., 2014; Mente et al., 2015; Tang et al., 2014; Tang et al., 2013; Troseid et al., 2015; Wang et al., 2011; Wang et al., 2014b). TMAO lowering interventions are thus of considerable interest for their potential therapeutic benefit (Brown and Hazen, 2015).
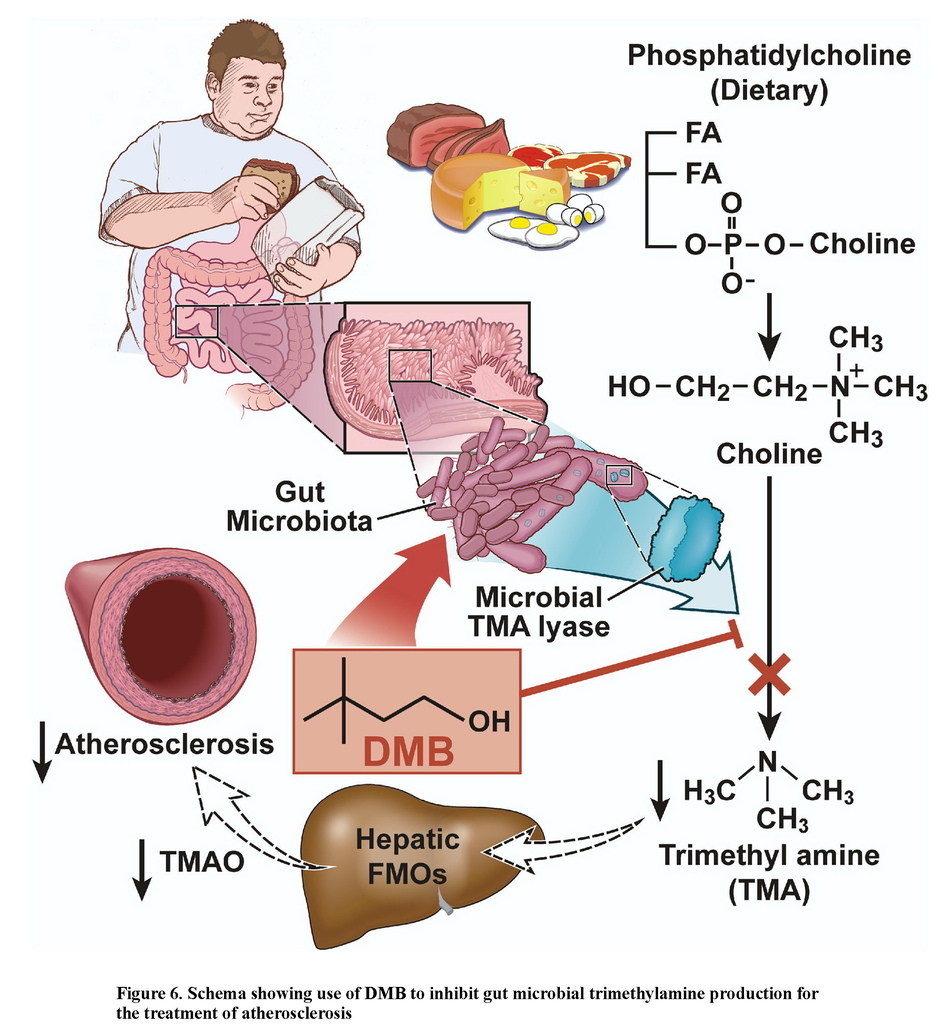
TMAO formation in mammals occurs via a two-step metaorganismal pathway (Koeth et al., 2013; Wang et al., 2011). Specifically, following nutrient ingestion, gut microbes form TMA, and then host hepatic flavin monooxygenases (FMOs) catalyze the conversion of TMA into TMAO (Koeth et al., 2013; Wang et al., 2011). Recent studies highlight the importance of both TMAO and host hepatic FMO3, the primary FMO responsible for TMAO production, as important regulators in host lipid and sterol metabolism, as well as atherosclerosis development (Bennett et al., 2013; Koeth et al., 2013; Shih et al., 2015; Wang et al., 2011; Warrier et al., 2015). In addition, recent microbial transfer studies confirm that both choline-diet enhanced atherosclerosis susceptibility and TMA/TMAO formation are transmissible traits (Gregory et al., 2015). Accordingly, the concept of “drugging the microbiome” to inhibit the metaorganismal pathway responsible for TMAO generation is of interest as a potential therapeutic approach for prevention of atherosclerosis. Toward that end, recent studies confirm that suppression of the host enzyme, FMO3, through antisense oligonucleotide-based approach, results in reduction of both circulating TMAO levels and diet-enhanced atherosclerosis in animal models (Miao et al., 2015; Shih et al., 2015). However, while numerous beneficial effects with FMO3 inhibition have been noted, FMO3 inhibition is also accompanied by several untoward side effects, including hepatic inflammation (Shih et al., 2015; Warrier et al., 2015). It is also well established that humans with genetic defects in FMO3 suffer from Fish Odor Syndrome due to the accumulation of TMA and its noxious odor (Cashman et al., 2003), reducing the appeal of FMO3 as a potential therapeutic target for inhibition. Reduction in TMAO levels by non-lethally targeting the gut microbial pathway(s) involved in TMA production has not yet been explored as a potential therapeutic approach to inhibit atherosclerosis.
RESULTS
Identification of an inhibitor of microbial TMA formation from choline
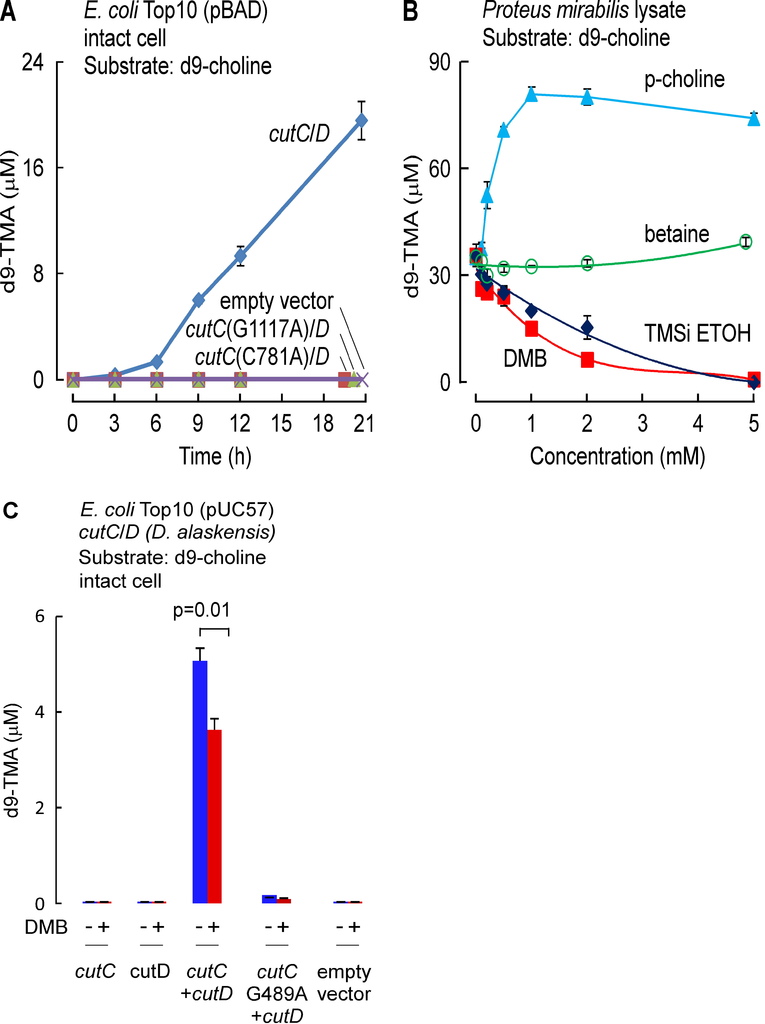
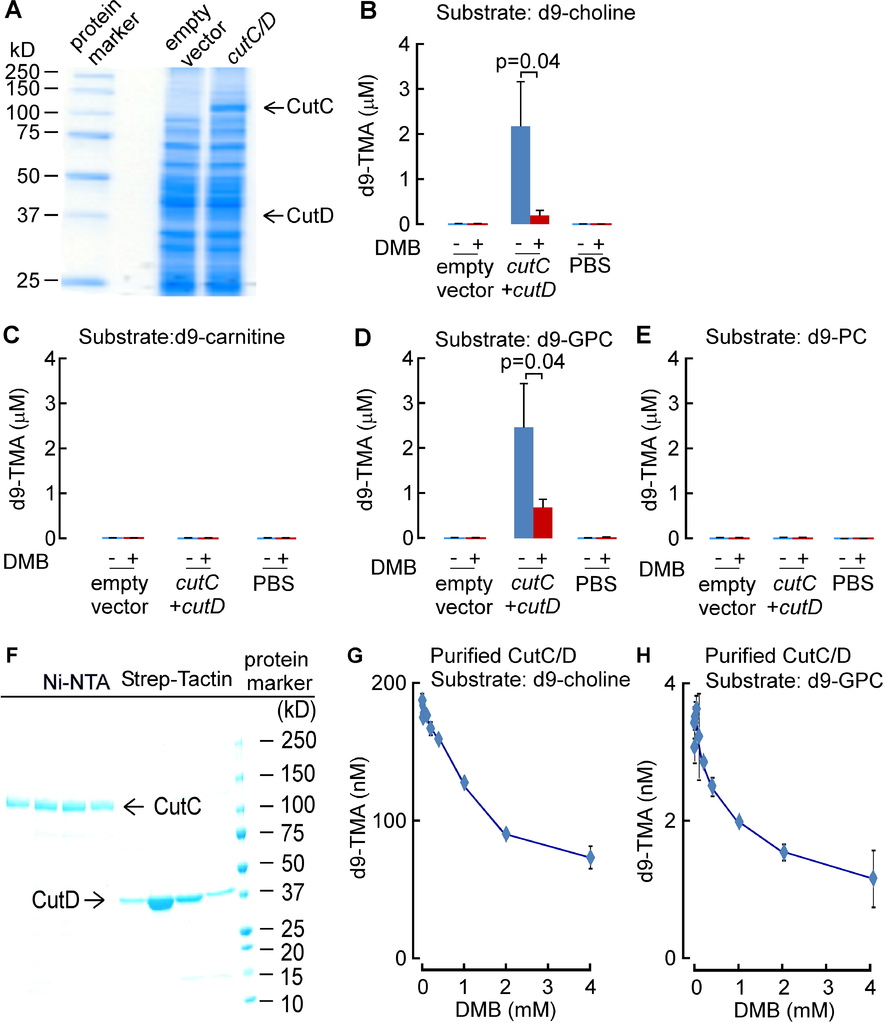
Anaerobic choline degradation is believed to serve as the major source of TMA formation within the intestines (Bain et al., 2005; Wang et al., 2011). We therefore focused initial drug screening efforts on identification of potential inhibitors of microbial TMA production from choline, or so-called choline-TMA lyase activity. The cleavage of the C-N bond of choline to form TMA and acetaldehyde was reported with extracts of Proteus mirabilis, a known human commensal microorganism that is easily cultured, nearly three decades ago (Sandhu and Chase, 1986). We therefore initially used P. mirabilis in early drug screening studies. However, during the course of our drug discovery efforts, the identification of a microbial choline TMA lyase was reported as a unique glycyl radical employing enzyme complex comprised of a catalytic polypeptide, CutC, and an associated activating protein, CutD, encoded by adjacent genes within a gene cluster (Craciun and Balskus, 2012). After this report, we subsequently cloned and expressed the cutC/D genes from P. mirabilis and confirmed that the expressed cutC/D gene complex serves as a microbial choline TMA lyase enzyme source (Figure S1A). For example, intact E. coliTop10 cells in culture lack choline TMA lyase activity, but following transformation with both P. mirabilis cutC/D genes, acquire capacity to enzymatically cleave choline to produce TMA. To further confirm that microbial TMA lyase enzyme activity monitored with this system required the presence of both CutC and CutD, multiple additional controls were examined, including the demonstration of lack of choline TMA lyase activity following transformation with only cutC or cutD individually, or following transformation with cutD but in combination with point mutants of cutC previously reported (Craciun and Balskus, 2012) to lack TMA lyase activity (cutC C781A or G1117A of P. mirabilis were made, which correspond to mutation of the conserved thiyl and glycyl-radical active site residues C489 and G821 in D. alaskensis) (Figure S1A).
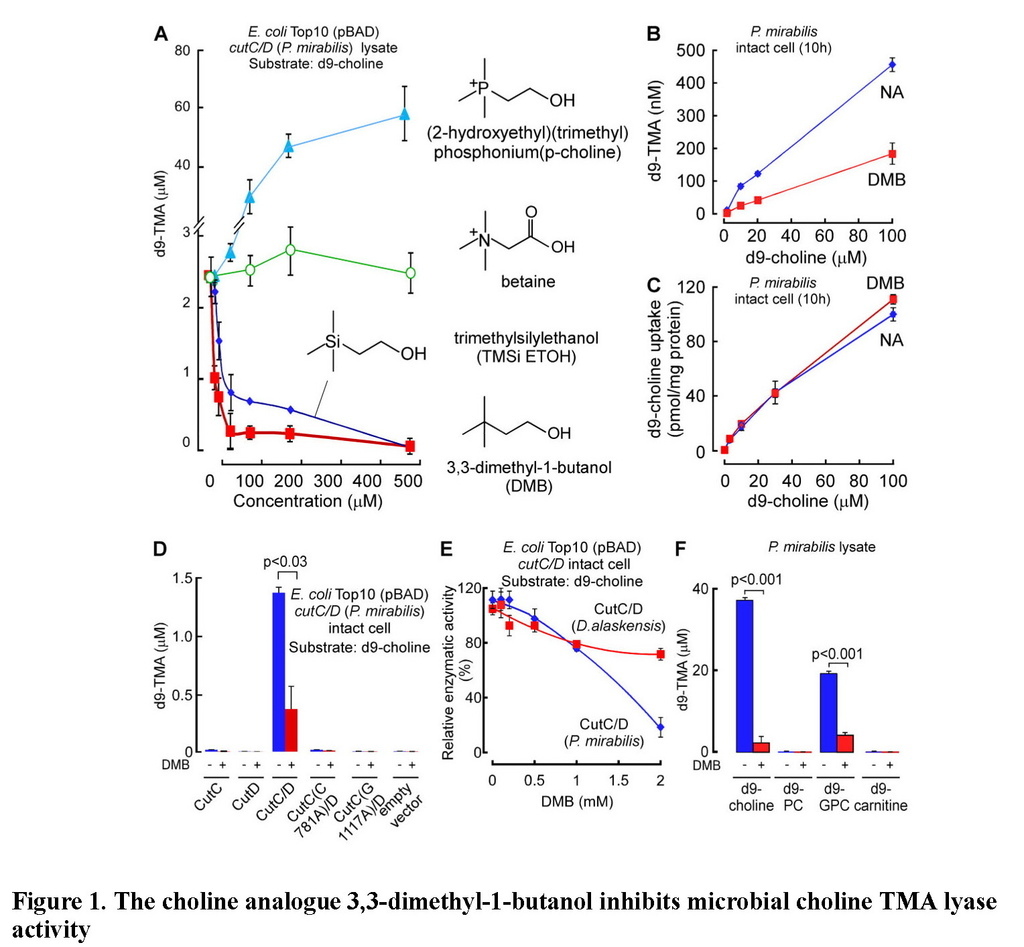
We surveyed multiple different natural and synthetic structural analogues of choline for their effect on the two microbial choline TMA-lyase activity systems (microbial TMA production using P. mirabilis lysate or recombinant choline TMA lyase activity using cutC/D from P. mirabilis transformed into E. coli) and noted three distinct patterns. Similar results were observed with both systems, with data shown in Figure 1Abeing from cutC/D transformed E. coli cell lysates, and illustrative data from P. mirabilis lysates shown in Figure S1B. The majority of choline analogues showed no effect on TMA lyase activity, which was monitored by quantifying the impact of the analogues on d9-trimethylamine (d9-TMA) formation from a fixed amount of [d9-N,N,N-trimethyl]choline (d9-choline) substrate. One notable example in this category is betaine (Figure 1A, Figure S1B), a choline oxidation metabolite and food component we recently showed can serve as a substrate for gut microbe-dependent TMA and TMAO generation in vivo, albeit at a rate approximately 100-fold less than that observed with a comparable amount of ingested choline (Wang et al., 2014b). Two of the choline analogues examined, 3,3-dimethyl-1-butanol (DMB) and 2-(trimethylsilyl)ethanol (TMSi-ETOH), where the nitrogen in choline is replaced with either a carbon or silicon atom, respectively, showed roughly comparable cutC/D choline TMA lyase inhibitory activity with an IC50 in the ~10 μM range in the recombinant cutC/D system (Figure 1A). Interestingly, the phosphonium analogue of choline (P-choline) showed a unique effect by augmenting cutC/D choline TMA lyase activity (Figure 1A, Figure S1B), suggesting this choline analogue acts as an agonist, and may promote more effective interaction between the catalytic (CutC) and activating (CutD) polypeptides. Of the choline TMA lyase activity inhibitors identified, we suspected DMB, given its structure, might be relatively non-toxic, and possibly even found as a natural product in existing foods or alcoholic beverages. Indeed, screening of multiple fermented liquids, oils and distilled products confirmed it is present in some alcoholic beverages and oils to varying levels (e.g. DMB was detected in some balsamic vinegars, red wines, and in some cold-pressed extra virgin olive oils and grape seed oils; highest levels observed of 25mM, data not shown). We thus elected to take DMB forward as a potential tool drug with which to test the hypothesis that inhibiting microbial production of TMA might serve as a potential therapeutic approach for the treatment of atherosclerosis.
(A) Effect of the indicated choline analogues on microbial TMA lyase activity (measured as d9-TMA production from 100 μM of the indicated d9-labeled substrate) from lysate of E. coli Top10 cells transformed (pBAD vector) with cutC/D genes (from P. mirabilis). (B) Effect of DMB on choline TMA lyase activity in intact P. mirabilis incubated with the indicated concentrations of d9-choline substrate ± DMB (1 mM) at 37°C. (C) DMB effect on choline uptake. P. mirabilis (OD600 nm = 0.5) were pelleted and then re-suspended in minimal media supplemented with the indicated concentrations of d9-choline ± DMB (2 mM) for 15 minutes at 37°C. Intracellular d9-choline was then quantified as described under Methods. (D) Choline TMA lyase activity from intact E. coli Top10 cells transformed with the indicated constructs in the presence vs. absence of DMB. (E) DMB dose response curves for inhibition of choline TMA lyase activity in intact E. coli Top10 cells transformed with cutC/D genes from either D. alaskensis (pUC57 vector) or P. mirabilis (pBAD vector). (F) TMA lyase activity for the indicated substrates in P. mirabilis lysate ± DMB. Data are presented as mean ± SE from three independent replicates (A–F). See also Figures S1, S2, S6.
In further in vitro studies, DMB was shown to block TMA production from live microbes, inhibiting the rate of intact P. mirabilis conversion of d9-choline into d9-TMA (Figure 1B). Moreover, parallel studies showed no effect of DMB on choline uptake by the live microbes, indicating that DMB does not block choline uptake into the cells (Figure 1C). Interestingly, while DMB inhibited choline TMA lyase activity of E. coli transformed with cutC/D from P. mirabilis (Figure 1D), the sensitivity of CutC/D from different microbial species to DMB inhibition varied. For example, transforming E. coli with cutC/D from D. alaskenesis, whose homologous CutC and CutD amino acid sequences show 63% and 47% identity to CutC/D from P. mirabilis, respectively, showed decreased extent of inhibition with DMB (Figure 1E; Figure S1C). An obligatory role of CutC in the enzymatic cleavage of choline to produce TMA (and DMB inhibition) in E. coli transformed with the cutC/D gene complex from either P. mirabilis or D. alaskensiswas supported in both cases by control studies where the respective cutC gene was mutated to replace previously reported (Craciun and Balskus, 2012) critical active site residues involved in catalysis (either C781A or G1117A for CutC from P. mirabilis (Figure 1D), or G489A for CutC from D. alaskensis (Figure S1C)). Examination of alternative trimethylamine substrates with the recombinant cutC/D system (E. colitransformed with P. mirabilis cutC/D) revealed DMB inhibited conversion of both d9-choline and [d9-N,N,N-trimethyl]glycerophosphocholine (d9-GPC) substrates into d9-TMA (Figure S2A–E). Moreover, when affinity tagged CutC and CutD (from E. coli Stellar cells transformed with P. mirabilis cutC/D) were individually isolated to homogeneity, addition of DMB to anaerobic reaction mixtures containing both purified polypeptides showed dose-dependent inhibition in TMA production from either choline or GPC substrates (Figure S2F–H). Further studies showed choline and GPC also serve as preferred substrates over [d9-N,N,N-trimethyl]phosphatidylcholine (d9-PC) and [d9-N,N,N-trimethyl]carnitine (d9-carnitine) for TMA production from P. mirabilis lysates, and in both instances, addition of DMB inhibited TMA formation (Figure 1F).
DMB inhibits some but not all microbial TMA lyases
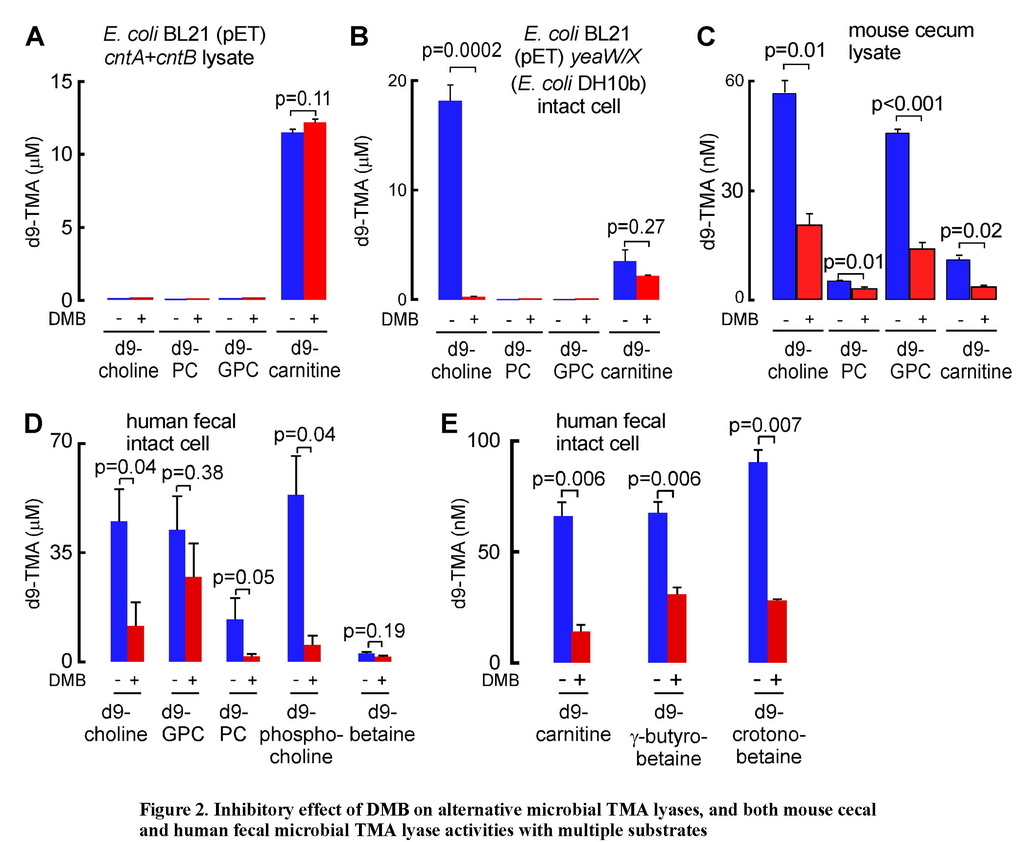
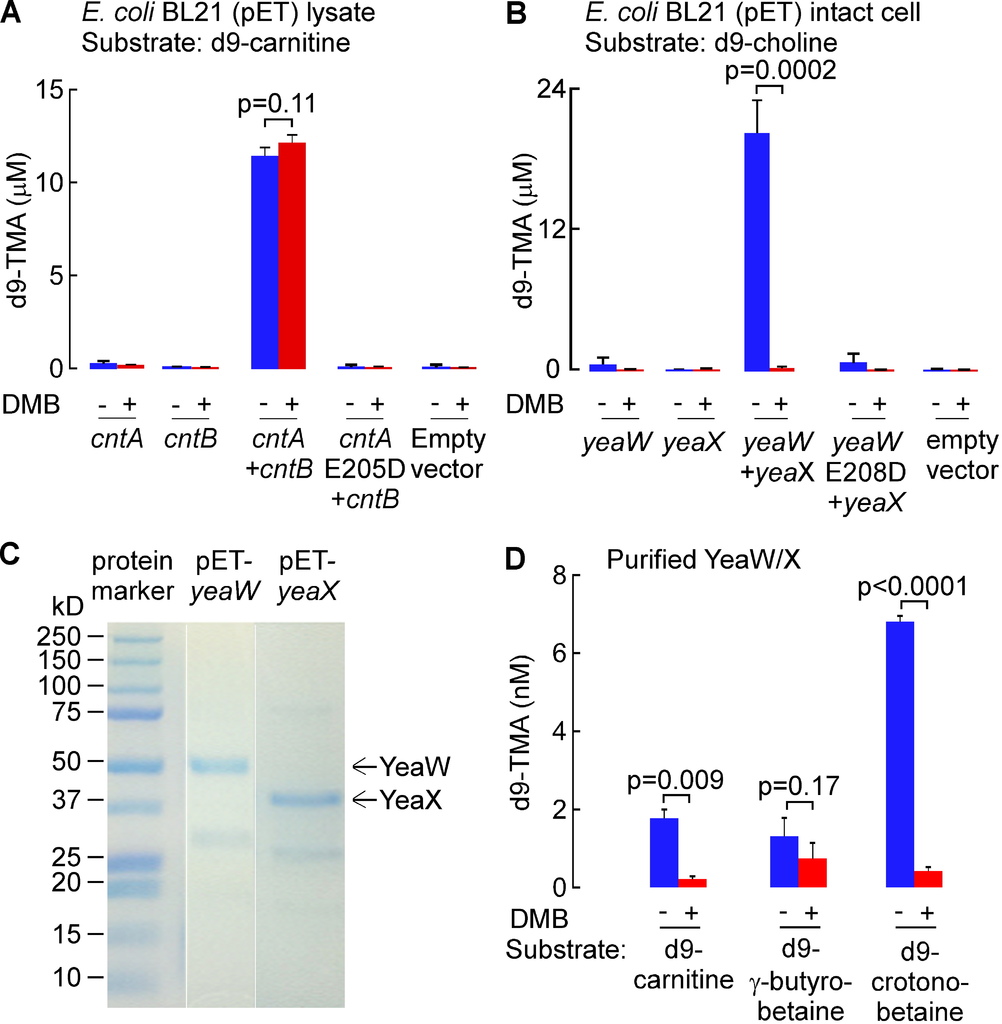
Since the CutC/D microbial TMA lyase complex does not effectively use either d9-carnitine or d9-PC as substrates (Figures S2C and S2E), yet both TMA-containing nutrients can be converted into TMA by microbes, we sought to explore whether DMB might serve as an inhibitor to alternative microbial TMA lyase activities. Toward this end, we first cloned the cntA (oxygenase) and cntB (reductase) genes from Acinetobacter baumannii, a recently reported microbial (carnitine-specific) TMA lyase enzyme complex that belongs to the Rieske-type iron-sulfur protein family (Zhu et al., 2014). Examination of wild-type E. coli BL21 strain showed no carnitine TMA lyase activity, so we transformed these cells with either cntA or cntB (from A. baumannii). While neither transformed E. coli possessed carnitine TMA lyase activity individually, combining the lysates from both cntA and cntB transformed E. coli showed the expected acquired enzymatic activity of cleaving d9-carnitine to form d9-TMA (Figure 2A; Figure S3A). Further confirmation that the carnitine TMA lyase activity required the CntA polypeptide was achieved by showing the lysate from E. coli transformed with a cntA point mutant previously shown to lack carnitine TMA lyase activity, E205D (Zhu et al., 2014), failed to show activity when combined with cntBtransformed E. coli lysates and incubated with d9-carnitine (Figure S3A). DMB showed no inhibitory effect on CntA/B catalyzed cleavage of d9-carnitine, and examination of the substrate preference of CntA/B revealed no evidence of TMA production in the presence of alternative potential precursor substrates such as choline, PC or GPC (Figure 2A).
(A) TMA lyase activity for the indicated substrates (375 μM each) in combined lysates from E. coli BL21 cells transformed (pET22 vector) with cntA and cntB genes (from A. baumannii) incubated in the presence or absence of DMB. (B) TMA lyase activity with the indicated substrates (375 μM, ± DMB) in intact E. coliBL21 cells transformed (pET22 vector) with yeaW/X genes (from E. coli DH10B). (C–E) TMA lyase activity with the indicated substrates (± DMB) incubated with (C) mouse cecum lysate (1.3 mg/ml protein) or (D–E) human fecal microbes (equivalent to 100 mg feces/ml). The DMB concentration in all reaction systems was 10 mM (in A, B) or 2 mM (C–E). Data are presented as mean ± SE from three independent replicates (A–E). See also Figures S3 and S6C,D.
We recently reported the cloning and characterization of an alternative TMA lyase enzyme complex from E. coli DH10B strain, YeaW/X, which also appears to be a two-component Rieske-type oxygenase/reductase that, at the amino acid level, shows 71% and 50% sequence homology to CntA and CntB, respectively (Koeth et al., 2014). Despite the homology to CntA/B, YeaW/X was found to possess broader substrate usage and could produce TMA from either choline or carnitine as substrate (Koeth et al., 2014). To examine the impact of DMB on YeaW/X TMA lyase activity, we first transformed E. coli BL21 cells, which show no detectable TMA lyase activity, with either or both of the yeaW/X genes. We observed that DMB inhibits choline TMA lyase enzyme activity in the presence of both YeaW and YeaX (Figure 2B; Figure S3B). Purified YeaW/X showed enzymatic cleavage of d9-carnitine, [d9-N,N,N-trimethyl]γ-butyrobetaine (d9-γ–butyrobetaine) and [d9-N,N,N-trimethyl]crotonobetaine (d9-crotonobetaine), producing d9-TMA. DMB inhibited carnitine and crotonobetaine, but not γ–butyrobetaine, conversion into TMA (Figure S3C–D).
DMB inhibits TMA formation from multiple substrates in physiological polymicrobial cultures
We next examined mouse cecal contents as a physiological polymicrobial source of TMA lyase activities with which to further characterize the potential inhibitory effect of DMB. Mouse cecal contents were homogenized and lysed under anaerobic conditions and then incubated with a fixed amount of individual potential d9-labeled TMA precursors (d9-choline, d9-PC, d9-GPC, or d9-carnitine) in the absence versus presence of DMB. All TMA containing nutrients examined served as substrates for TMA production by the polymicrobial enzyme source. Moreover, addition of DMB inhibited TMA formation from each substrate, suggesting broad inhibitory activity across multiple microbial TMA lyase enzyme activities (Figure 2C). In a further set of studies, we used human feces from healthy donors as another physiological polymicrobial source of TMA lyase activity. Intact fecal bacteria were recovered and then incubated with a fixed amount of individual d9-labelled potential nutrient precursors (d9-choline, d9-GPC, d9-PC, [d9-N,N,N-trimethyl]phosphocholine (d9-phosphocholine), [d9-N,N,N-trimethyl]betaine (d9-betaine), d9-carnitine, d9-γ-butyrobetaine and d9-crotonobetaine) in the absence versus presence of DMB. Both the substrate preference and the inhibitory effect of DMB on human fecal microbial TMA production were assessed. First, all nutrient precursors examined produced TMA, with greater extent of production from nutrients structurally related to choline (Figure 2D) compared to carnitine (Figure 2E). Moreover, DMB significantly inhibited TMA production from each substrate except for betaine and GPC, both of which showed a trend toward inhibition that was not statistically significant (Figures 2D and 2E).
DMB is a non-lethal inhibitor of TMA production by microbes in a rich nutrient environment
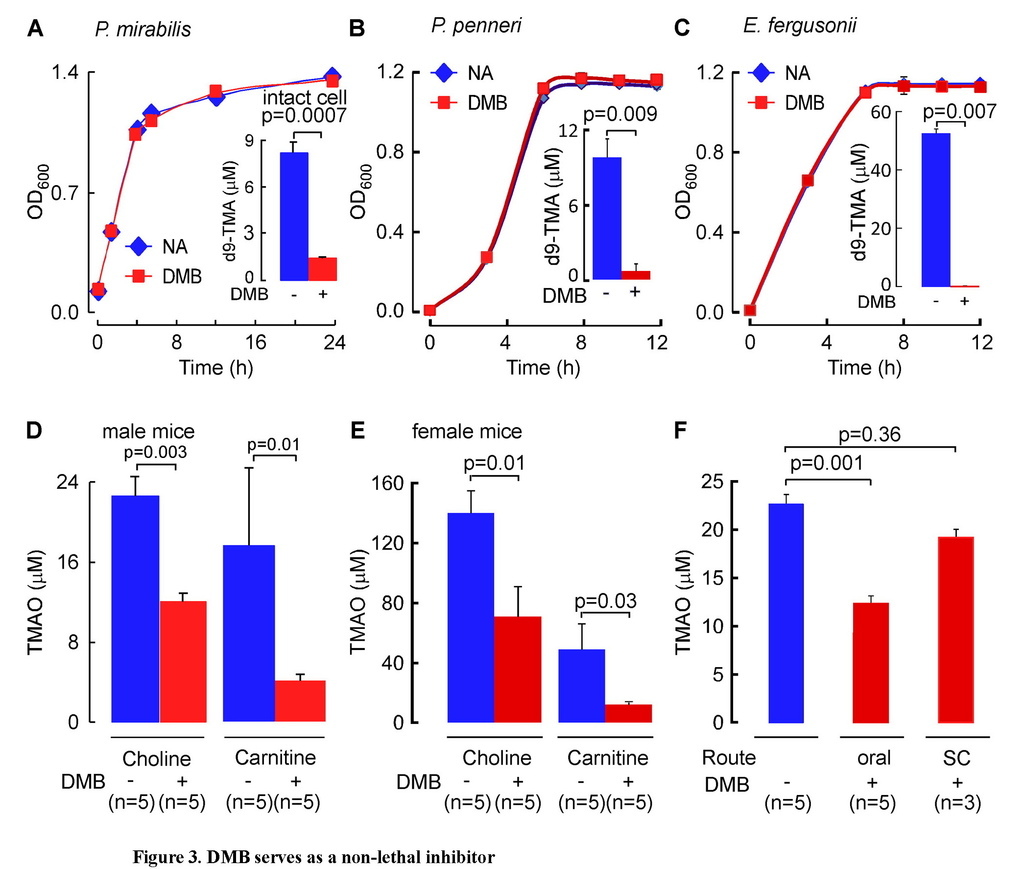
Importantly, DMB was shown to serve as a non-lethal inhibitor (i.e., not an antibiotic) in cultures of P. mirabilis grown in a rich nutrient broth under conditions that showed no adverse impact on cell growth despite marked inhibition in microbial TMA lyase activity (Figure 3A). Recently, several human bacterial isolates were identified that are capable of producing TMA from choline in culture, as well as in vivo when used to colonize germ-free mice (Romano et al., 2015). We therefore explored the impact of DMB on TMA production and bacterial growth using some of these additional human commensals. Both Proteus penneri and Escherichia fergusonii were confirmed to possess choline TMA lyase activity, readily consuming choline and producing TMA. Similar to results observed with P. mirabilis, addition of DMB to a rich nutrient broth in cultures (as exists in the gut lumen) of either Proteus penneri or Escherichia fergusonii markedly inhibited choline utilization to produce TMA but showed no inhibitory effect on bacterial growth (Figures 3B and 3C).
(A–C) Cells (A, P. mirabilis; B, P. penneri; C, E. fergusonii) were grown in nutrient broth ± DMB (0.1% v/v) and both the growth rate (OD600) and total amount of d9-TMA produced from d9-choline (100 μM) (insert) were monitored. (D–E) Demonstration that DMB decreases plasma TMAO levels in male (D) and female (E) C57BL/6J Apoe−/− mice placed chronically on either 1.0% choline or 1.0% carnitine-supplemented diets in the presence versus absence of DMB (1%, v/v in drinking water). (F) Three groups of male C57BL/6J Apoe−/−mice (8 week old) were placed on chemically defined diet equivalent to chow supplemented with choline (1.0% total choline). Two groups of mice were administered 1.2 μmol of DMB daily (provided in corn oil, 150 μl total vol) by either (oral) gastric gavage, or subcutaneous (SC) routes, and the third group of mice received sham (vehicle) gavages daily. After two weeks, plasma TMAO levels were determined by LC/MS/MS. Data are presented as mean ± SE from three (A–C) or the indicated numbers (D–F) of independent replicates.
See also Figure S4C and Tables S1 and S2.
DMB lowers plasma TMAO levels in vivo
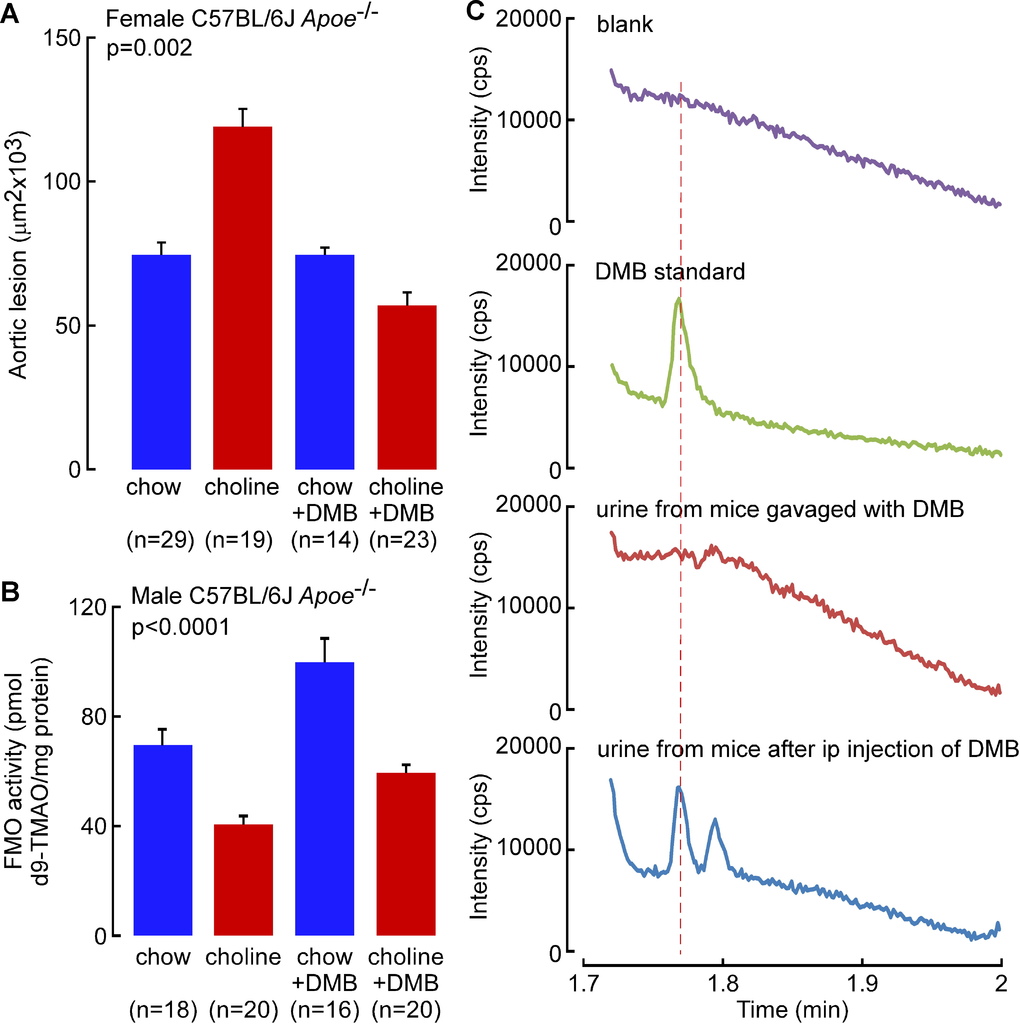
To examine the impact of DMB on microbial TMA production in vivo, several studies were performed. First, different groups of C57BL/6J mice were placed on chemically defined diets identical in composition except for supplementation with either choline (total choline 1.0%, w/w) or carnitine (1.0%, w/w), diets previously shown to raise TMAO levels and accelerate atherosclerosis in mice (Koeth et al., 2013; Wang et al., 2011). In addition, at the start of the study, each dietary group of mice was further divided by exposing half to DMB, provided in the drinking water. Provision of DMB resulted in significant reduction in plasma TMAO levels in both the choline-supplemented and the carnitine-supplemented groups, with similar results observed for both male and female mice (Figures 3D and 3E). In further studies, comparison of the effect of route of administration revealed that while orally administered DMB resulted in significant reduction in plasma TMAO levels, a comparable amount of DMB administered subcutaneously failed to reduce plasma TMAO levels (Figure 3F). Next, atherosclerosis prone apolipoprotein E null mice (Apoe−/−) at time of weaning were placed chronically (16 weeks) on the same chemically defined diets comparable to chow (0.07 % total choline) versus the same diet supplemented with choline (1.0 % total choline) in the presence versus absence of DMB provided in the drinking water. Examination of plasma TMAO levels among the groups of mice showed that while TMAO was markedly elevated in the mice on the choline-supplemented diet (11.8-fold, p<0.001), addition of DMB again substantially inhibited the choline diet-induced increase in plasma TMAO concentrations (mean ± SD, in μM, for the four groups: chow, choline, chow + DMB and choline + DMB, are 1.02 ± 0.09, 22.1 ± 2.1, 0.69 ± 0.06, and 15.9 ± 0.9, respectively; p<0.01 for comparison of choline vs. choline + DMB). Moreover, mice showed no evidence of toxicity to the chronic (16 week) DMB exposure, with no significant increases in circulating lipid levels, plasma choline, glucose or insulin levels, and normal indices of both renal (creatinine) and liver function (transaminase activities, bilirubin) (Table S1). Further tissue biochemical studies confirmed chronic exposure to DMB did not adversely impact liver lipid (cholesterol, cholesteryl ester and triglyceride) levels, and histology (including Oil-Red-O and H&E) staining showed no signs of steatohepatitis (data not shown).
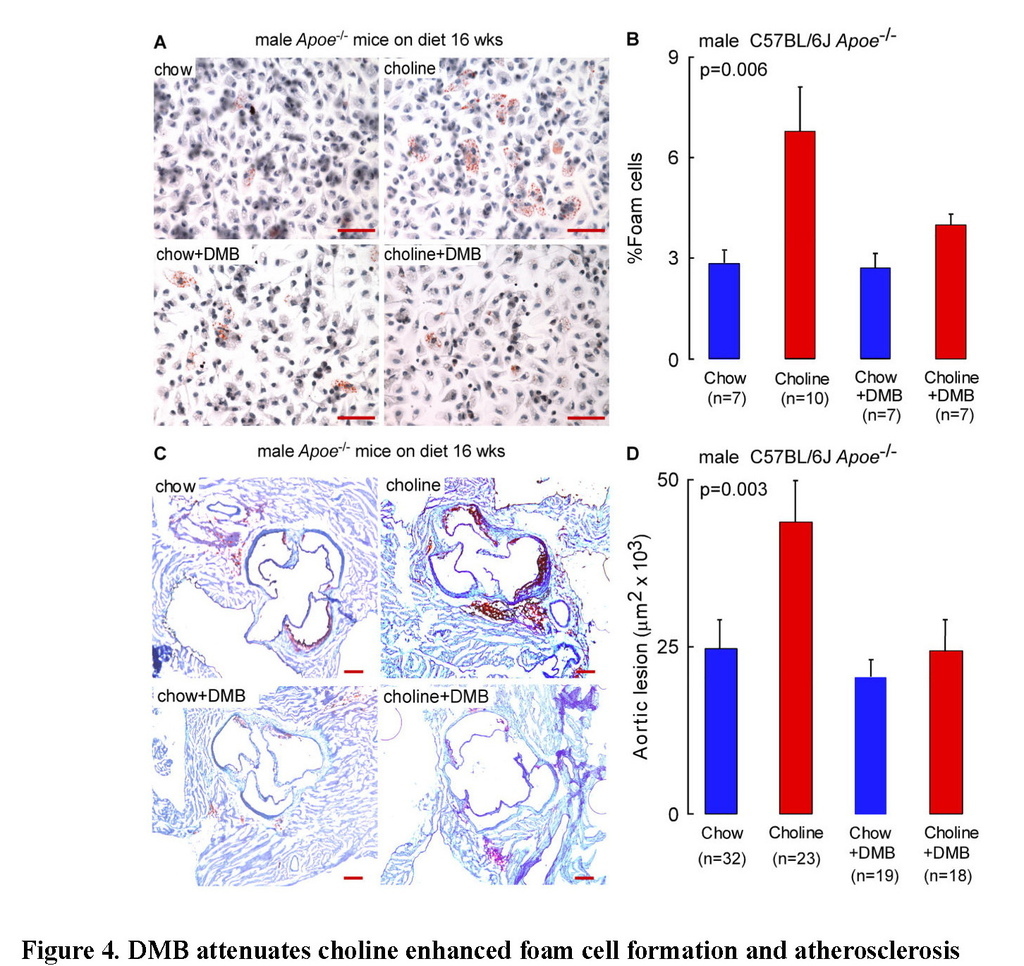
DMB attenuates choline diet-enhanced macrophage foam cell formation and atherosclerosis
A high total choline content is a characteristic feature of a Western diet. We previously reported that dietary choline supplementation enhanced macrophage cholesterol accumulation, foam cell formation, and atherosclerosis in Apoe−/− mice via microbial TMA/TMAO formation (Wang et al., 2011). The effect of chronic DMB exposure (in the drinking water) on endogenous peritoneal macrophage foam cell formation was therefore next examined in the Apoe−/− mice maintained chronically (16 weeks from time of weaning) on the chow (0.07 % total choline) versus choline-supplemented (1.0 % total choline) diets. As was previously observed (Wang et al., 2011), a choline-enriched diet promoted marked increase in endogenous macrophage foam cell generation (approximately 2-fold on choline-supplemented diet; p=0.02), yet addition of DMB to the drinking water significantly reduced the observed diet enhanced macrophage foam cell formation (p<0.05 for comparison of choline vs. choline + DMB; Figures 4A and 4B). To assess the impact of DMB on high choline diet-dependent enhancement in atherosclerosis in the Apoe−/− mice, parallel studies in male and female mice were performed in similarly treated Apoe−/− mice maintained chronically (16 weeks) from time of weaning on the chemically defined chow (0.07 % total choline) versus choline-supplemented (1.0 % total choline) diets, in the presence versus absence of DMB (in drinking water). Comparable results (DMB mediated attenuation of atherosclerosis) were observed in both male and female mice (p=0.003 and p=0.01, respectively), with data from males shown in Figures 4C and 4D, and results with females shown in Figure S4A. Briefly, aortic root lesion plaque quantification revealed that mice on a chronic, choline-supplemented diet, as expected, showed significant enhancement in atherosclerotic plaque area (approximately 2-fold increase; p=0.003 males, Figures 4C and 4D; p=0.03 females, Figure S4A) despite no atherogenic changes in plasma lipids (Table S1). Remarkably, addition of DMB significantly inhibited the choline diet-dependent enhancement in aortic root lesion development in both males (p=0.006, Figure 4D) and females (p=0.02, Figure S4A). Since observed reductions in plasma TMAO in DMB treated Apoe−/− mice could also occur via changes in hepatic FMO activity, we also examined total hepatic FMO enzymatic activity in liver homogenates generated from the different groups of mice. Contrary to what one might expect with the reduced TMAO plasma levels, mice exposed to DMB showed not a reduction, but instead, a modest but statistically significant increase in total FMO hepatic enzyme activity (p<0.0001, Figure S4B).
(A) Representative Oil-Red-O/hematoxylin staining of peritoneal macrophages, Scale bar, 50 μm, and (B) foam cell quantification, from 20 week old male C57BL/6J Apoe−/− mice fed chemically defined chow (0.07% total choline) or 1% choline-supplemented diets from time of weaning (4 weeks). Data are mean ± SE. (C) Representative Oil-Red-O/hematoxylin staining of aortic root sections from 20 week old male C57BL/6J Apoe−/− mouse fed chemically defined chow (0.07% total choline) or choline-supplemented (1.0% total choline) diets in the presence vs. absence of DMB provided in the drinking water, as described under Experimental Procedures. Scale bar, 200 μm. (D) Aortic root lesion area was quantified in 20 week old male C57BL/6J Apoe−/− mice from the indicated diet and DMB treatment groups. Data are mean ± SE. p values shown were calculated by ANOVA.
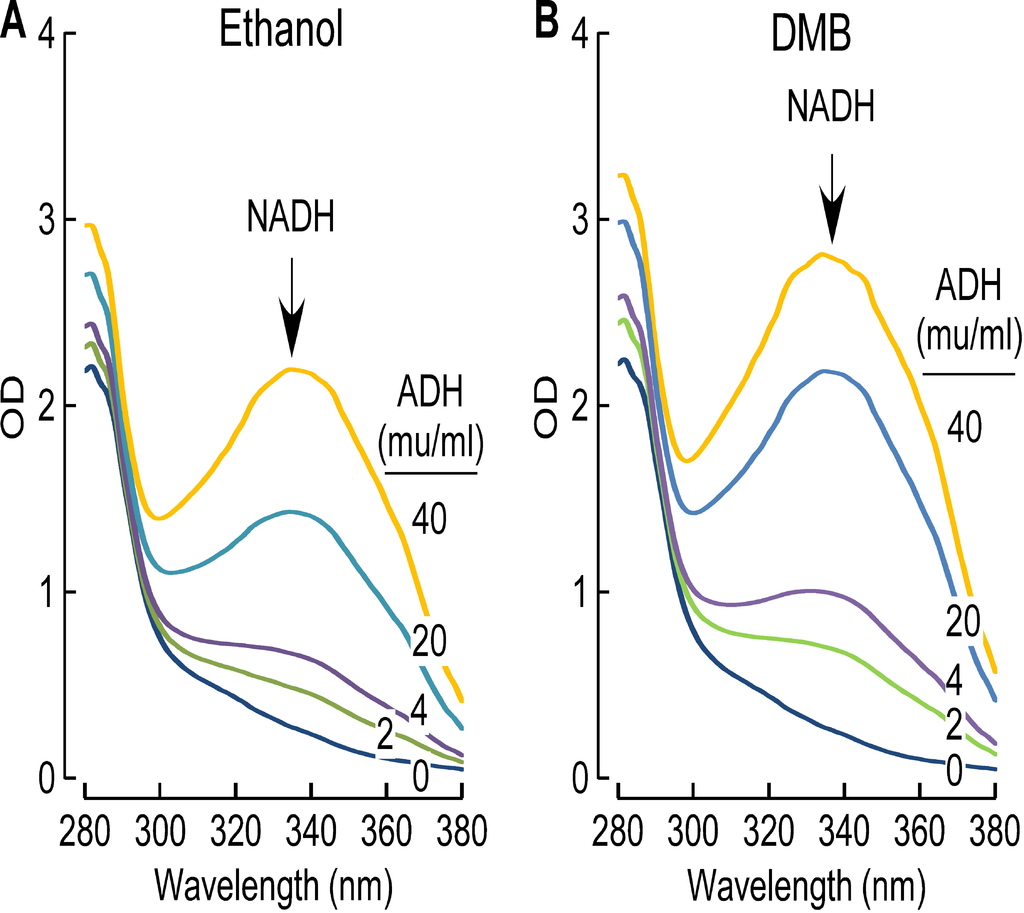
Despite extensive efforts we were unable to detect DMB in the plasma or urine of mice chronically exposed to DMB in the drinking water. Moreover, when equivalent amounts of DMB were provided to mice either via intraperitoneal, or oral route, DMB was detected in both plasma and urine 30 minutes following intraperitoneal route, but not following bolus administration by gastric gavage (Figure S4C). This suggested the possibility that very rapid hepatic metabolism of the agent may account for the low systemic circulating levels observed following oral administration, absorption and portal blood delivery of DMB to the liver. Consistent with this hypothesis, DMB was found to be rapidly metabolized by liver alcohol dehydrogenase at a rate comparable to the substrate ethanol (Figures S5A and S5B). We could not detect the initial predicted metabolite, 3,3-dimethylbutyric aldehyde, in plasma or urine, but instead found evidence of further rapid metabolism to the carboxylic acid, with detection of the metabolite, 3,3-dimethylbutyrylcarnitine, in urine (structure confirmed by high resolution mass spectrometry; data not shown).
It has previously been reported that 30 minutes after intraperitoneal administration of DMB, through competitive inhibition of choline dehydrogenase, the choline content of liver tissues increases (Barlow and Marchbanks, 1985). Choline dehydrogenase is expressed mainly in the liver and kidney and catalyzes conversion of choline into betaine aldehyde, an intermediate in the generation of betaine (Cafiero, 1951; Tan et al., 1981). In additional studies, we therefore measured levels of choline, betaine, TMA and TMAO in liver and kidney, as well as plasma and urine, from C57BL/6J Apoe−/− mice chronically administered DMB in the drinking water. No evidence of tissue choline elevation was noted in either liver or kidney following chronic oral DMB treatment in mice; however, modest elevations in plasma and kidney betaine levels were observed in mice on choline diet with DMB (vs. choline diet without DMB) (Table S2). Notably, as expected with provision of an inhibitor of microbial TMA production, the most significant effects detected with DMB exposure were reductions in TMA and TMAO levels in all tissues and fluids examined (liver, kidney, plasma and urine), especially under conditions of choline diet supplementation (Table S2).
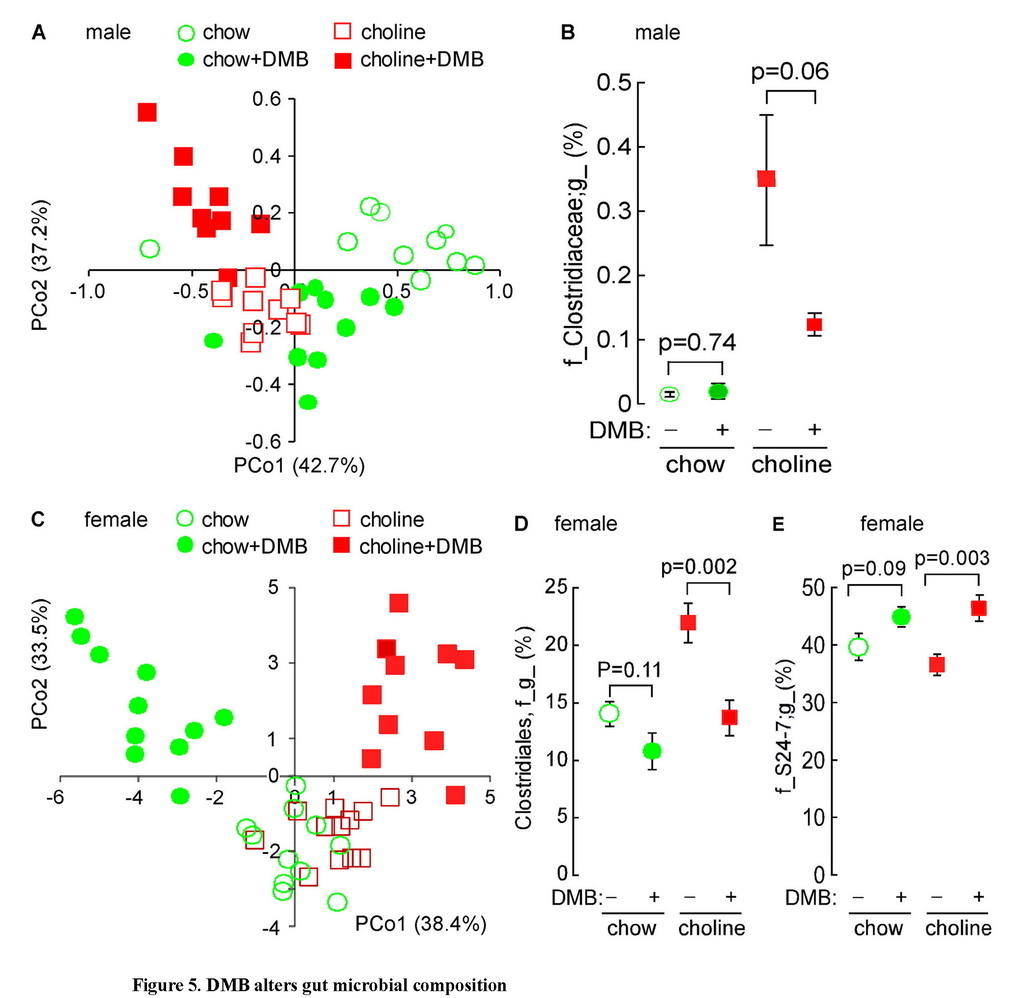
DMB reduces the proportions of some taxa associated with TMAO levels
One theoretical advantage of a therapeutic agent that functions as a non-lethal, microbial enzyme-targeted inhibitor, as opposed to an antibiotic, is that there should be less selective pressure for development of resistance. Nonetheless, the gut microbiome is known to be remarkably dynamic and adaptive, including changes in response to different dietary inputs, as well as the presence versus absence of distinct diseases (Cox et al., 2014; Koren et al., 2011; Wu et al., 2011). We therefore sought to examine whether chronic exposure to DMB may alter TMAO levels not only via inhibition in microbial TMA production, but also by potentially inducing reorganization of the gut microbial community. Cecal microbial compositions were analyzed in both male and female mice in the atherosclerosis studies by sequencing 16S rRNA gene amplicons in the various Apoe−/− mice groups. Global analyses of community structure showed differences between the chow (0.07% choline) versus high choline (1.0%) diet fed groups, which became more pronounced in the presence of DMB (Figures 5A and 5C). Further examination revealed the proportions of several taxa within all groups of mice within each gender were associated with aortic root lesion area and plasma TMA and TMAO levels to some extent (Tables S3 and S4). For example, among male mice, proportions of the taxa Clostridiaceae were highly positively correlated with plasma TMA and TMAO levels, as well as atherosclerotic lesion area (Table S3). Further, male mice placed on a choline-supplemented diet showed significant increase in the cecal proportion of this taxon, and exposure to DMB promoted a strong tendency (p=0.06) to decrease the proportion of this taxon in mice fed a choline-supplemented diet (Figure 5B).
Unweighted UniFrac distances plotted in PCoA space comparing cecal microbial communities in male (A) or female (C) C57BL/6J Apoe−/− mice fed chow versus choline-supplemented diet in the presence versus absence of DMB. Each data point represents a sample from a distinct mouse projected onto the first two principal coordinates (percent variation explained by each PCo is shown in parentheses). (B, D, E) Impact of diet and DMB on the proportion of several taxa. Data represent mean ± SE (n ≥ 9 mice per group).
See also Tables S3 and S4.
For female mice, the order Clostridiales and the genus Ruminococcus were each highly positively correlated with both plasma TMA and TMAO levels, as well as atherosclerotic lesion area (Table S4). Similarly, prevalence of the taxon Lachnospiraceae was highly associated with TMA and TMAO levels and showed a trend toward increased aortic root plaque area. It is also interesting to note that the proportion of the taxa Clostridiales, the most abundant bacterial order observed in cecum, was positively correlated to plasma TMA levels (r=0.35, p=0.03), and many Clostridiales species have been reported to cleave choline (Moller et al., 1986). Further, female mice placed on a choline-supplemented diet showed a significant increase in the proportions of this taxon, and exposure to DMB induced a significant decrease in the proportions of this taxon (Figure 5D; Table S4). In contrast, proportions of S24-7, an abundant family from Bacterioidetes, showed significant inverse associations with both atherosclerotic plaque area and TMA levels and a trend toward reduced plasma TMAO (Figure 5E; Table S4). Indeed, within the female mice, examination of the impact of DMB on multiple taxa associated with TMA or TMAO levels and atherosclerotic plaque extent indicates that provision of the agent induced a reduction in the levels of many bacterial taxa positively associated with levels of TMA, TMAO, or aortic root lesion area, and conversely, a rise in the proportions of many of the bacterial taxa negatively associated with TMA, TMAO or aortic lesion area (Table S4).
Discussion
Recent advances identify a pivotal role for gut microbiota in multiple phenotypes associated with cardiometabolic diseases. A causal role for the involvement of gut microbes is in part based on the fulfillment of one of Koch’s postulates - namely, the transmission of a disease related phenotype with transplantation of gut microbial organisms to a new host (Cho and Blaser, 2012; Cox et al., 2014; Dumas et al., 2006; Gregory et al., 2015; Koch, 1880; Sandhu and Chase, 1986). The recognition of gut microbes as participants in cardiovascular and metabolic disease pathogenesis suggests pharmacological interventions aimed at “drugging the microbiome” may potentially serve as a therapeutic approach for the treatment of cardiometabolic diseases. Toward this end, in the absence of knowledge of specific microbial enzyme activities or their metabolites mechanistically linked to disease phenotypes, therapeutic approaches, aside from dietary restrictions, have thus far instead focused on altering gut microbial community composition through use of either defined microbial compositions (probiotics) or non-microbial substances that may secondarily foster alteration in microbial community structure (prebiotics) (Petschow et al., 2013). Multiple lines of evidence show the meta-organismal pathway involved in TMAO production is mechanistically linked to atherosclerosis susceptibility (Bennett et al., 2013; Gregory et al., 2015; Koeth et al., 2013; Wang et al., 2011). However, the effect of small molecule antagonists for the critical initiating microbial TMA lyase step on atherosclerosis development has not yet been reported.
The present studies provide proof of concept for the idea that inhibiting microbial TMA production, through microbial TMA lyase inhibition, may serve as a potential therapeutic approach for the prevention or treatment of atherosclerosis (Figure 6). DMB, a structural analogue of choline, served as a preliminary tool compound to inhibit microbe-dependent TMA and host TMAO formation from multiple distinct TMA containing nutrients in vitro and in vivo. Moreover, DMB provision, despite no significant effects on circulating cholesterol, choline and other pro-atherogenic risk factors, inhibited choline diet-dependent accumulation of both macrophage cholesteryl ester accumulation (foam cell formation) and aortic root atherosclerotic plaque development. In addition to attenuating plasma levels of TMAO, DMB promoted reduction in proportions of some microbial taxa that are associated with plasma TMA and TMAO levels, and aortic plaque extent. While the impact of DMB on microbial composition was noticeable, and one might anticipate that there is less selective pressure for development of resistance against a non-lethal enzyme inhibitor compared to an antibiotic, the fact that there is any change in microbial composition induced by DMB suggests some degree of selective pressure is occurring with exposure to the agent, and thus raises the possibility of development of resistance.
DMB (3,3-dimethyl-1-butanol) is a structural analogue of choline, and an inhibitor of microbial TMA production (a choline TMA lyase inhibitor). Provision of DMB in the drinking water of atherosclerosis prone Apoe−/− mice inhibits choline diet dependent enhancement in TMAO, endogenous macrophage foam cell formation, and atherosclerosis development.
Gut microbes serve as a filter for our largest environmental exposure–what we eat. They also function, fundamentally, as a dynamic endocrine organ, generating biologically active metabolites in response to specific nutrient inputs that can enter the circulation and elicit biological effects at distant sites within the host (Brown and Hazen, 2015). The present studies suggest development of selective, non-lethal microbial enzyme inhibitors that target causative pathways, once they are revealed, may serve as an effective and complementary strategy to current approaches for the prevention and treatment of cardiometabolic diseases.
EXPERIMENTAL PROCEDURES
More complete experimental procedures are provided in Supplemental Experimental Procedures.
General procedures and reagents
Reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated. Routine blood tests were measured on a Roche Cobas Analyzer (Indianapolis, IN). Insulin was measured by ELISA with Mouse Insulin ELISA kit (Mercodia, Uppsala, Sweden). Protein concentration was determined by BCA assay. Hepatic FMO activity was quantified using liver homogenate and d9-TMA substrate, monitoring NADPH-dependent production of d9-TMAO, as described previously (Bennett et al., 2013).
Ethical considerations
All animal model studies were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic. All study protocols and informed consent for human subjects were approved by the Cleveland Clinic Institutional Review Board. Informed consents were obtained for all subject samples.
Chemical syntheses
P-choline [(2-Hydroxyethyl)(trimethyl)phosphonium] was synthesized by reaction of trimethylphosphine and chloroethyl alcohol, using a modification of methods described previously (Renshaw and Bishop, 1938). d9-PC, d9-γ-butyrobetaine and d9-crotonobetaine and d9-carnitine were synthesized as previously described (Koeth et al., 2014; Koeth et al., 2013; Tang et al., 2013; Wang et al., 2011). d9-GPC was synthesized by reacting glycerophosphoethanolamine (Corden Pharma, Cambridge, MA) with CD3I and purified by silica gel column chromatography (Koeth et al., 2013). Structural characterization of all synthetic material can be found in Supplemental Experimental Procedures.
Mass spectrometry quantification of analytes
Stable isotope dilution liquid chromatography with on-line tandem mass spectrometry (LC/MS/MS) was used for quantification of plasma and tissue TMAO, choline and betaine as previously described (Wang et al., 2011; Wang et al., 2014a). Samples used for TMA quantification were acidified prior to storage, and used d9-TMA as an internal standard by LC/MS/MS. Liver cholesterol was quantified by gas chromatography mass spectrometry (GC/MS) (Wang et al., 2011). DMB was quantified by GC/MS from plasma/urine after extraction with hexane. Cellular uptake of d9-choline was quantified from washed intact bacteria following freeze-thaw cycle induced lysis by LC/MS/MS using [1,1,2,2-d4]choline as internal standard. Complete details can be found in the Supplemental Experimental Procedures.
Determination of TMA lyase activity
TMA lyase activity was typically quantified by incubating (10–16h) the indicated enzyme source [intact cell (OD600 nm ~ 1.0), cell lysate (3 mg protein) or purified protein (30 μg)] with the indicated co-factors in the presence or absence of DMB (2 mM) unless otherwise stated. Reactions were initiated by addition of d9-labeled substrate (100 μM, unless otherwise indicated) in gas-tight reaction vials at 37°C. d9-TMA production was quantified by LC/MS/MS. Complete details can be found in the Supplemental Experimental Procedures.
Cloning and Expression of cutC/cutD, cntA/cntB and yeaW/yeaX
P. mirabilis (ATCC 29906) whole genome sequence, contig00092 (ACLE01000068.1) was used to acquire the nucleotide sequence of cutC (locus tag: HMPREF0693_2863 ), cutD (locus tag: HMPREF0693_2862) and the 104 bases of intergenic DNA between cutC/D. The open reading frames (ORFs) for cutC/D were codon optimized, synthesized contiguously by GenScript (Piscataway, NJ) and then cloned into pBAD (Invitrogen, Carlsbad, CA) and transformed into E. coli Top10 cells (Invitrogen). For expression of amino-terminal 8x-His-tagged CutC and carboxy-terminal Strep-Tag II-tagged CutD, the codon optimized cutC-intergenic region-cutD from P. mirabilis was PCR amplified and cloned in-frame into the plasmid pGK::nucMCS that was isolated from L. lactis (ATCC® 8731™) behind an N-terminal 8x-His tag and in front of a Strep-tag II affinity tag. Sequence verified clones were transformed into E. coli Stellar cells (Clontech, Moutainview, CA). The ORFs for Desulfovibrio alaskensis G20 cutC/D genes (Ddes_3282 and Ddes_1357, respectively) and their associated intergenic region were codon optimized for expression in E. coli and synthesized by Genscript for cloning into pUC57. Constitutive expression of the D. alaskensisG20 CutC/D proteins in E. coli Top10 cells (Invitrogen), was performed using the synthetic em7constitutive bacterial promoter with ribosome binding site, as described under Supplemental Experimental Procedures. The genes for yeaW/yeaX were cloned from E. coli K-12 DH10B genomic DNA by PCR, inserted into a modified pET22 vector backbone and expressed in E. coli BL21(DE3) (Invitrogen) (Koeth et al., 2014). For expression of 8x-His-tagged YeaW and 8x-His-tagged YeaX proteins, the relevant genes were cloned in-frame behind an 8x-His-tag in E. coli BL21(DE3). CntA/cntB genes from Acinetobacter baumannii (ATCC 19606) (Zhu et al., 2014) were codon optimized and synthesized (Genescript), and then cloned into pUC57, inserted separately into a modified pET22 vector backbone, and expressed as un-tagged proteins in E. coli BL21(DE3), as described in Supplemental Experimental Procedures. The catalytically inactive C489A and G821A mutants in D. alaskensis G20 CutC (Craciun and Balskus, 2012), C781A and G1117A mutants of P. mirabilis (ATCC 29906) CutC, and E205D mutant of cntA Acinetobacter baumannii (ATCC 19606) (Zhu et al., 2014), and E208D mutant of E. coli K-12 DH10B YeaW were all made by site-directed mutagenesis as described in Supplemental Experimental Procedures.
Growth and induction conditions for recombinant TMA lyases
Recombinant clones transformed in E. coli Top10 and BL21(DE3), were grown in LB medium with 100 μg/ml ampicillin, and arabinose or isopropyl β-D-1-thiogalactopyranoside (IPTG) was added at a final concentration of 0.2% or 0.4 mM for induction of TMA lyase subunits constructed in pBAD or pET vectors, respectively. The cutC/D recombinant clones in the pGK or pUC57 vectors were grown in LB medium with erythromycin (250 μg/ml) or ampicillin (100 μg/ml), respectively, at 37°C overnight to constitutively express the targeted TMA lyase subunit. Further details can be found in the Supplemental Experimental Procedures.
Mouse models
For atherosclerosis and endogenous foam cell formation studies, at the time of weaning (4 weeks of age), apolipoprotein E knockout mice (C57BL/6J Apoe−/− ;>40 generations back-crossed) were fed either a chemically defined standard chow (Teklad 2018) (total choline 0.07%) or the same diet supplemented to 1.0% choline (Teklad TD.07864) (total choline confirmed by LC/MS/MS). Where indicated, mice were also treated with DMB (1.0%, v/v) and/or L-carnitine (1.0%) added to the drinking water. Aortic lesion quantitation was performed as described (Baglione and Smith, 2006; Wang et al., 2011). Endogenous macrophage foam cell formation was assayed following peritoneal lavage collection 72 h after 4% thioglycollate elicitation (Wang et al., 2007).
Bacterial, mouse cecum and human feces TMA lyase sample preparation
Mouse cecum from C57BL/6J Apoe−/− mice or human feces was gently homogenized, followed by centrifugation under argon atmosphere. The upper phase was collected followed by centrifugation under argon blanket. The bottom pellet was collected, resuspended, and used as intact bacterium cell suspensions under either nitrogen or argon atmosphere in gas-tight vials. Lysates of bacteria were generated either by French Press using argon gas, or by homogenization with 100 μm glass beads using a TissueLyser II (Qiagen, Valencia, CA) kept within an argon or nitrogen filled chamber.
Microbiota studies
Bacterial DNA was isolated from mouse cecum and 16S rRNA gene V4 hypervariable regions were amplified, pyrosequenced, assigned into different taxa and analyzed as previously described (Gregory et al., 2015; Koeth et al., 2013). Principal component analysis (PCA) was conducted using MarkerView Software (ABSciex, Framingham, MA 01701) to compare the effects of different diets and DMB exposure on microbiota composition.
Statistical analyses
Data were analyzed with Student’s t test and ANOVA with post hoc t test with Holm-Bonferroni correction to compare difference in mean value between different groups for most studies, as indicated. Pearson’s correlation was used to calculate the association between two variables after demonstration of normal distribution of data. For all statistical tests p < 0.05 was considered significant.
Supplementary Material
1
2
3
4
5
6
7 Click here to view.(174K, pdf)
8. Click here to view.(32K, docx)





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 