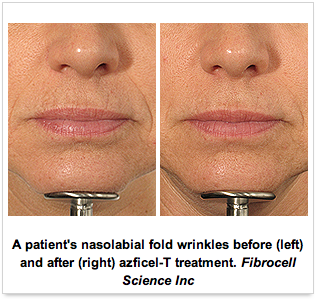
The US Food and Drug Administration (FDA) late yesterday approved the first autologous aesthetic cell therapy to improve the appearance of moderate to severe nasolabial fold wrinkles in adults.
The product, azficel-T (laVív), is from Fibrocell Science, Inc, a company focused on developing personalized cell therapies for aesthetic, medical, and scientific applications.
According to the company, creating azficel-T involves a patented technology whereby fibroblasts are extracted from behind the patient's ear and sent to the Fibrocell Science laboratory, where they are multiplied for about 3 months and then frozen until needed.
Over a series of 3 treatment sessions, typically 3 to 6 weeks apart, they are injected into nasolabial folds to reduce the appearance of smile lines.
The company says the therapy will become available gradually through trained clinical investigators in select metropolitan areas. As manufacturing capacity is increased and more physicians trained, the number of cities served will expand as well.
The product will be mostly administered by dermatologists and plastic surgeons; only physicians who complete a Fibrocell-approved training program will be able to administer it.
The training program teaches proper biopsy collection and shipment procedures, treatment preparation and injection technique, and logistics tracking to ensure that the cells received by each patient are derived from that same patient.
"Revolutionary" Approach
"The concept of using a patient's own collagen-making cells is a revolutionary way to help treat nasolabial fold wrinkles and help restore a fresh appearance," Robert A. Weiss, MD, clinical associate professor, Johns Hopkins School of Medicine, Baltimore, and director of the Maryland Laser Skin and Vein Institute, Hunt Valley, noted in a company-issued statement.
"Since this is a biological process that works over time, [azficel-T] is able to provide gradual and natural-looking results," noted Dr. Weiss, who participated in clinical trials of azficel-T.
This approach is "likely appeal to patients who want to take a very new approach to treating wrinkles," Stacy Smith, MD, associate clinical professor in the Division of Dermatology at the University of California–San Diego, who also worked on azficel-T clinical trials, said in a statement.
"By injecting tens of millions of the person's own fibroblasts, patients now have the option to help smooth smile lines by adding cells to replace those that may have been lost through the aging process," he added.
Babak Azizzadeh, MD, FACS, director of the Center for Advanced Facial Plastic Surgery and assistant clinical professor of facial plastic surgery at University of California–Los Angeles, commented, "It's an interesting and novel approach that will generate some excitement among physicians and some patients.
"But at the end of the day," he told Medscape Medical News, "it's going to depend on whether the results are better than with off-the-shelf injectables, such as Juvederm (Allergan), Restylane (HA North American Sales AB), and Sculptra (Sanofi-Aventis), which are the main competition."
Dr. Azizzadeh was not involved in the studies of azficel-T and has not treated any patients with it.
He added that "a limiting factor is that the patients have to have a biopsy and then wait 3 months before they get their injections, and then they have to go through a series of treatments. It's a long process. Physician acceptability, with the length of time involved, may also be an issue."
2 Pivotal Clinical Trials
The FDA approval was based largely on 2 identical phase 3 multicenter, randomized, double-blind, placebo-controlled studies involving 421 patients who underwent 3 treatment sessions approximately 5 weeks apart.
On the basis of investigators' and patients' assessments, a significantly greater proportion of patients demonstrated a positive response to treatment with azficel-T than with placebo, the company notes. The treatment improved the appearance of nasolabial fold wrinkles for the 6 months of patient follow-up after the last treatment. The company said studies are ongoing, looking at how long beyond 6 months after the last treatment the effect may last.
In clinical trials, azficel-T was well tolerated, according to the company. The most common adverse events were mild to moderate injection-site reactions that usually resolved within 1 week. As part of a postmarketing requirement, Fibrocell will set up a registry of approximately 2700 patients to further evaluate the safety of this autologous cell therapy.