Volume 362:1312-1324
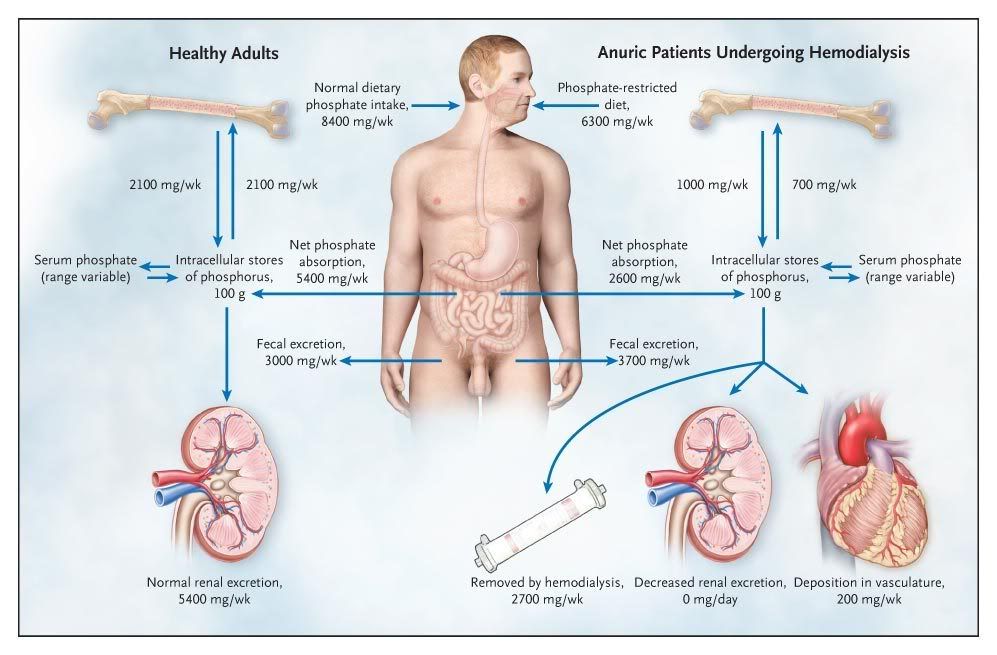
Figure 1. Phosphate Metabolism in Kidney Failure and in Health.
In healthy adults, phosphate intake is matched by phosphate excretion in feces and urine, and the flux of phosphate between the skeleton and the extracellular phosphate pool is approximately the same in both directions. In patients with kidney failure, dietary restriction of phosphate is insufficient to compensate for the decrease in renal phosphate excretion, resulting in a positive phosphate balance. In addition, bone is often resorbed more rapidly than it is formed because of abnormal bone remodeling in kidney failure. Together, these abnormalities may confer a predisposition to vascular calcification, especially when serum phosphate levels are suboptimally controlled. The phosphate values shown are for illustrative purposes only, since these values vary from patient to patient.
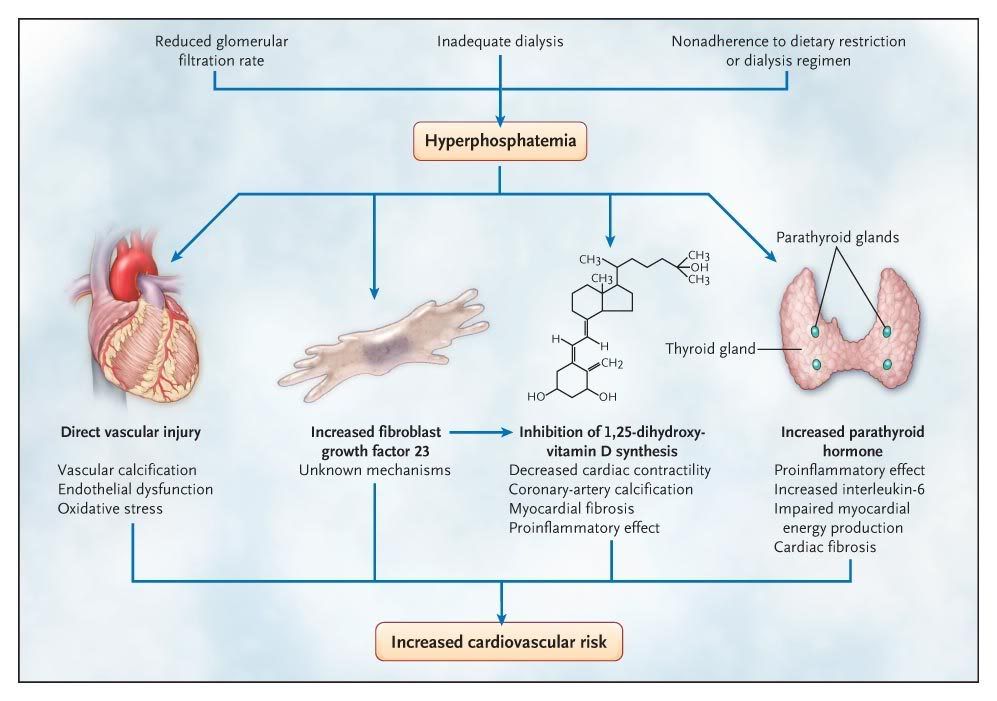
Figure 2. Putative Mechanisms Linking Hyperphosphatemia and Cardiovascular Disease.
Elevated serum phosphate levels are associated with an increased risk of cardiovascular disease among patients with and those without kidney failure, although it is unclear whether phosphate plays a causal role or is simply a marker of a poor outcome. Although much of the research has focused on the role of elevated phosphate levels in vascular calcification, multiple potential mechanisms linking phosphate to cardiovascular disease have been proposed. Hyperphosphatemia may also directly affect vascular health by increasing reactive oxygen species, thereby causing oxidative damage and endothelial dysfunction. Indirectly, hyperphosphatemia increases levels of parathyroid hormone and fibroblast growth factor 23, both of which have been suggested to have direct pathogenic cardiovascular effects. Increased phosphate levels have also been associated with inhibition of 1,25-dihydroxyvitamin D synthesis, which is associated with vascular calcification and myocardial disease. Finally, hyperphosphatemia might also identify patients who are less likely to comply with dietary restrictions (and other aspects of their care), which could confer a predisposition to cardiovascular disease.
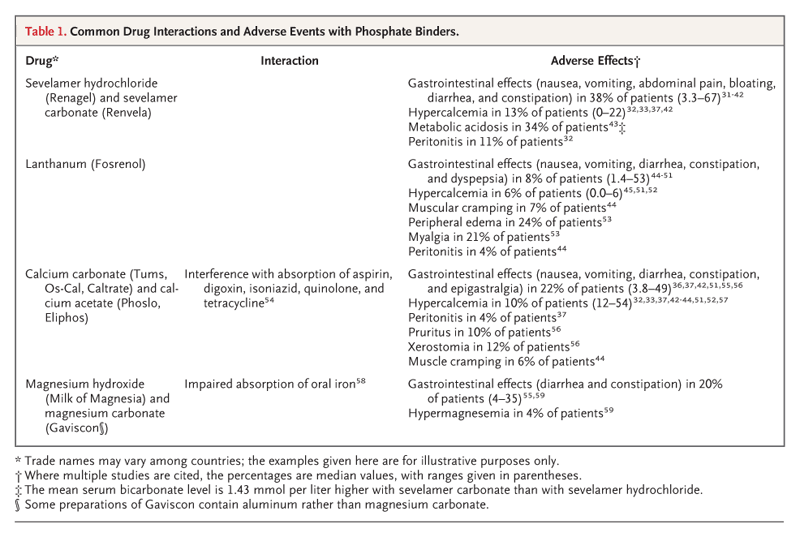
另外我摘錄一些藥物的介紹給各位看看:
Calcium-Based Phosphate Binders 鈣離子磷結合劑
Calcium-based phosphate binders, either calcium carbonate or calcium acetate, have been used for decades in patients undergoing dialysis, and the two agents appear to have relatively similar phosphate-binding ability per gram of calcium administered.26,27 They are the most commonly used phosphate binders in contemporary practice worldwide.
Sevelamer Renagel(R)磷能解
Sevelamer is an anion-exchange resin, first released as sevelamer hydrochloride, and almost all the clinical studies of sevelamer have used this formulation. Given concerns about metabolic acidosis due to the hydrochloride moiety, however, sevelamer is currently approved by the Food and Drug Administration and marketed as sevelamer carbonate, which appears to have a similar effect on phosphate lowering but has been much less extensively studied. Sevelamer use was reported in 17.1% of an international hemodialysis cohort (1996–2008), although current use in the United States appears to be substantially higher.
Lanthanum 鑭
Lanthanum carbonate is a nonaluminum, noncalcium phosphate-binding agent. Four short-term trials have compared lanthanum with placebo, and three trials have compared lanthanum with calcium-based binders. Four of the trials were double-blind, placebo-controlled studies that lasted from 4 to 6 weeks, two were open-label studies that lasted up to 1 year, and one was an open-label comparison of lanthanum with standard therapy (largely calcium-based phosphate binders) that lasted for 2 years. Withdrawal rates in the longer-term trials were high,with rates of 71% among lanthanum recipients in the largest trial, including 14% and 16% of the recipients who were withdrawn because of adverse events and those who withdrew consent, respectively.
Magnesium-Based Phosphate Binders 鎂離子磷結合劑
Although oral magnesium has been used as a phosphate binder for many years, relatively few data are available concerning its efficacy and safety. Serum magnesium levels are higher in patients undergoing dialysis than in persons with normal kidney function, and hypermagnesemia with respiratory arrest has been reported after excessive oral magnesium ingestion in such patients. Accordingly, most contemporary hemodialysis programs severely restrict or avoid the administration of medications that contain magnesium.
No studies evaluating magnesium-based binders have measured clinical end points or bone histologic features. In a randomized trial of 46 patients receiving hemodialysis, monotherapy with magnesium carbonate, in conjunction with a lower magnesium concentration in the dialysate (0.3 mmol per liter), was compared with calcium carbonate. Serum phosphate levels and the risk of adverse events were similar in the two groups during this 6-month study, although calcium recipients had lower levels of parathyroid hormone. Similar findings were reported when magnesium-based agents were used together with calcium-based binders, although in this study, hypercalcemia was less common in the combination-therapy group. Taken together, the results of these studies and others suggest that magnesium binders may have theoretical advantages over calcium-based binders that are similar to the advantages of other non–calcium-based binders, including a reduced calcium load. However, all these studies were small and of short duration. In the absence of long-term data on safety and efficacy, oral magnesium cannot currently be recommended for first-line use as a phosphate binder.
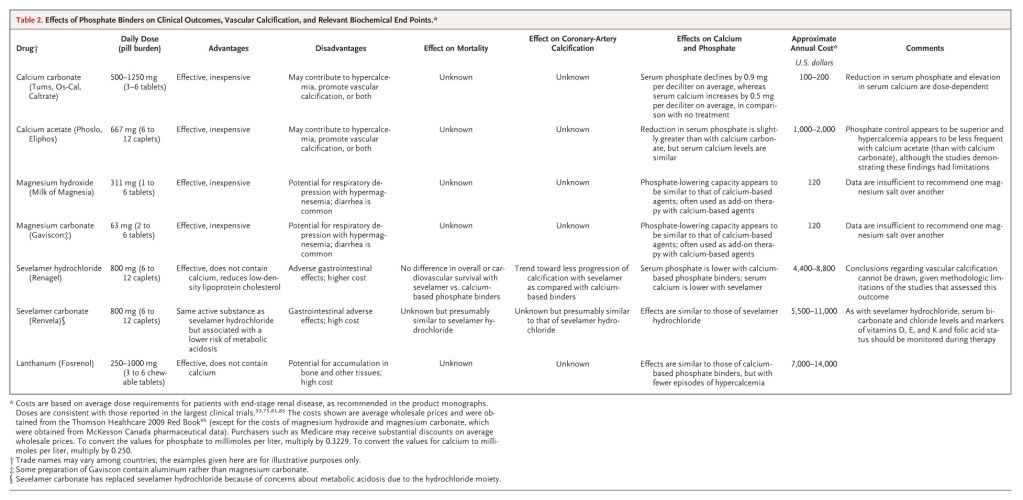
Suggestions for Management of Hyperphosphatemia
Dietary phosphate restriction effectively reduces serum phosphate levels and should be encouraged for all patients with end-stage renal disease. Although the optimal method of facilitating adherence to a phosphate-restricted diet is unknown, more frequent patient contact with renal dietitians may be beneficial, at least initially. In addition, specific counseling about the need to avoid phosphate-containing food additives leads to a substantial reduction in serum phosphate, as compared with the usual education about foods that are naturally high in phosphate. Even with careful dietary modification, most patients undergoing dialysis will continue to require oral phosphate binders. Given the potential dangers of using unvalidated surrogate end points to support the treatment choice, the selection of a particular phosphate binder cannot be justified solely on the basis of its effects on hyperphosphatemia or vascular calcification. Rather, we consider calcium-based agents to be the first-line phosphate binders for patients undergoing dialysis, since these preparations remain the least expensive and best tolerated option for the treatment of hyperphosphatemia. Although the optimal starting dose has not been studied, many clinicians initially prescribe 200 mg of elemental calcium with each meal for these patients.
Sevelamer and lanthanum are promising, but their superiority to calcium-containing agents has not been proved. Furthermore, they are expensive and are associated with more adverse events. In the absence of their proven clinical benefit as compared with calcium-based agents, in our view sevelamer and lanthanum cannot be recommended as initial therapy. Although the use of these drugs to supplement or replace calcium-based agents in patients with severe vascular calcification has been advocated,25 this strategy has not been tested in clinical trials. For patients in whom phosphate levels cannot be controlled with calcium-based agents alone (especially patients with hypercalcemia), short courses of magnesium-based binders are an inexpensive alternative. However, careful monitoring of serum magnesium levels is required (perhaps in conjunction with a lower magnesium concentration in the dialysate), and the long-term safety and efficacy of this approach are unknown. Some physicians may prefer to use sevelamer or lanthanum despite their higher cost.
Since the ideal target level of serum phosphate is not known, the initial goal of therapy should be to reduce the level until it approaches the normal range, recognizing that normophosphatemia will be unattainable for most patients. Whether other agents should be added to improve phosphate control should be considered in light of the severity of concomitant hyperparathyroidism, the patient's pill burden, the risk of adverse events, the cost of additional treatment, and the absence of definitive evidence of a benefit of tight phosphate control.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 