
Cameron P. Simmons, Ph.D., Jeremy J. Farrar, M.D., Ph.D., Nguyen van Vinh Chau, M.D., Ph.D., and Bridget Wills, M.D., D.M.
N Engl J Med 2012; 366:1423-1432
Dengue is a self-limited, systemic viral infection transmitted between humans by mosquitoes. The rapidly expanding global footprint of dengue is a public health challenge with an economic burden that is currently unmet by licensed vaccines, specific therapeutic agents, or efficient vector-control strategies. This review highlights our current understanding of dengue, including its clinical manifestations, pathogenesis, tests that are used to diagnose it, and its management and prevention.
DETERMINANTS OF THE CURRENT DENGUE PANDEMIC
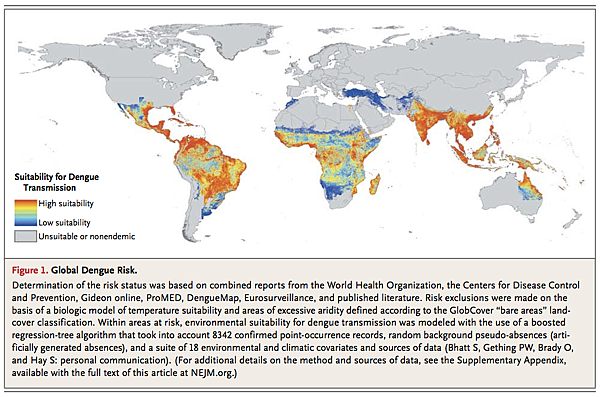
The global burden of dengue is large; an estimated 50 million infections per year occur across approximately 100 countries, with potential for further spread (Figure 1).
1 Central to the emergence of dengue as a public health problem has been the dispersal of efficient mosquito vectors across much of the tropical and subtropical world. The primary vector, the urban-adapted Aedes aegypti mosquito, has become widely distributed across tropical and subtropical latitudes. It emerged from Africa during the slave trade in the 15th through 19th centuries, spread into Asia through commercial exchanges in the 18th and 19th centuries, and has spread globally with the advent of increased travel and trade in the past 50 years.2 In addition, the geographic range of a secondary vector, A. albopictus, has dramatically expanded in recent years.3 Globalization of trade, in particular the trade of tires from used vehicles, is thought to explain the dispersal of eggs and immature forms of these arboviral vectors into new territories.4 Endemicity has also been facilitated by rapid urbanization in Asia and Latin America, resulting in increased population density with an abundance of vector-breeding sites within crowded urban communities and the areas surrounding them. Dengue infections in Africa remain largely unquantified, but recent outbreaks suggest that substantial parts of the continent may be at risk for increasing dengue transmission. More surveillance is required to assess the true burden of disease (see the Supplementary Appendix, available with the full text of this article at NEJM.org).
Vector control, through chemical or biologic targeting of mosquitoes and removal of their breeding sites, is the mainstay of dengue prevention, but this approach has failed to stop disease transmission in almost all countries where dengue is endemic. Antigenic diversity of the dengue virus is important, since the lack of long-term cross-immunity among the four virus types allows for multiple sequential infections.
Thus, the spread of dengue illustrates how global trade (and the transport of the mosquito vectors), increasing travel within and between countries (and the movement of viremic people), urban crowding (which is conducive to multiple infections from an infected mosquito), and ineffective vector-control strategies have supported a pandemic in the modern era. With the increasingly global spread of dengue, practicing physicians in temperate North America, Europe, Australia, and Japan are more likely than ever to see returning travelers with dengue infection. The diagnosis should be considered in any patient presenting with fever that has developed within 14 days after even a brief trip to the tropics or subtropics, including those regions where dengue has not traditionally been considered an endemic disease.5,6
VIROLOGIC FEATURES
Dengue is caused by one of four single-stranded, positive-sense RNA viruses (dengue virus type 1 through dengue virus type 4), also referred to as serotypes) of the genus flavivirus (family Flaviviridae). Infectious virus and the virus-encoded NS1 are present in blood during the acute phase, and high-level early viremia and NS1 antigenemia have been associated with more severe clinical presentations.7-9 The detection of NS1 is also the basis for commercial diagnostic assays.10
Dengue viruses exist in two environments: the urban or endemic setting, where humans and mosquitoes are the only known hosts, and forested areas, where transmission of mosquito-borne viruses occurs between nonhuman primates and, rarely, from these primates to humans.11 Within each dengue virus serotype, multiple genotypes comprise phylogenetically related sequences. Subtle antigenic differences exist between genotypes of the same serotype,12,13 but these may not be clinically relevant, since human infection with one serotype is believed to confer long-lived serotype-specific immunity, but only short-lived cross-immunity between serotypes.
The dynamics of dengue viruses within urban and endemic populations are complex, involving the birth and death of viral lineages.14,16 Although dengue has emerged in multiple new territories over the past 40 years, the viruses themselves are paradoxically “local” in their evolutionary histories, suggesting that the global dispersal of dengue virus has occurred in relatively infrequent “jumps,” most likely by the movement of viremic humans to new geographic settings with a suitable vector and a susceptible population.
IMMUNOPATHOGENESIS
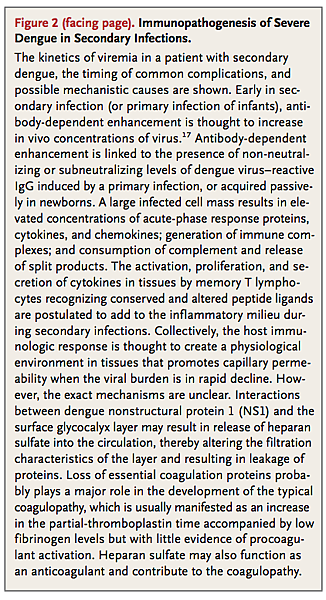
Insights into the pathogenesis of severe dengue are hampered by the lack of an animal model that accurately recreates the transient capillary permeability syndrome accompanied by a decreasing viral burden that is seen in patients (Figure 2).
Epidemiologic studies have identified young age, female sex, high body-mass index, virus strain, and genetic variants of the human major-histocompatibility-complex class I–related sequence B and phospholipase C epsilon 1 genes as risk factors for severe dengue.18-21 Secondary infection, in the form of two sequential infections by different serotypes, is also an epidemiologic risk factor for severe disease.17,22,23 Mechanistically, increased risk in secondary infection is thought to be linked to antibody-dependent enhancement of virus infection in Fc receptor–bearing cells and the generation of a large infected cell mass in vivo.24 A consequence of a large virus-infected cell mass is a physiological environment in tissues that promotes capillary permeability; however, this hypothesis is based on temporal associations between immunologic markers and clinical events, without evidence of a direct, mechanistic link to causation (Figure 2).
PATHOPHYSIOLOGY OF ENDOTHELIAL DYSFUNCTION
There is no evidence that the virus infects endothelial cells, and only minor nonspecific changes have been detected in histopathological studies of the microvasculature.25,26 Although no specific pathway has been identified linking known immunopathogenic events with definitive effects on microvascular permeability, thromboregulatory mechanisms, or both, preliminary data suggest that transient disruption in the function of the endothelial glycocalyx layer occurs.27,28 This layer functions as a molecular sieve, selectively restricting molecules within plasma according to their size, charge, and shape. Hypoalbuminemia and proteinuria are observed during dengue infection; proteins up to and including the size of albumin are preferentially lost; this is consistent with a small but crucial change in the filtration characteristics of the glycocalyx.29 Both the virus itself and dengue NS1 are known to adhere to heparan sulfate, a key structural element of the glycocalyx, and increased urinary heparan sulfate excretion has been detected in children with severe infection.30,31
DIFFERENTIAL DIAGNOSIS AND DISEASE CLASSIFICATION
Although most dengue virus infections are asymptomatic, a wide variety of clinical manifestations may occur, ranging from mild febrile illness to severe and fatal disease.1 The differential diagnosis is broad and varies as the disease evolves. During the febrile phase, it includes other arboviral infections as well as measles, rubella, enterovirus infections, adenovirus infections, and influenza. Other diseases that should be considered as part of the differential diagnosis, depending on the clinical picture and local disease prevalence, include typhoid, malaria, leptospirosis, viral hepatitis, rickettsial diseases, and bacterial sepsis.
Patients were previously classified as having either dengue fever or dengue hemorrhagic fever, with the latter classified as grade 1, 2, 3, or 4. Over a number of years, there was increasing concern regarding the complexity and usefulness of this classification system. In particular, there was concern regarding the requirement that all four specific criteria (fever lasting 2 to 7 days, tendency to hemorrhage evidenced by a positive tourniquet test or spontaneous bleeding, a platelet count of less than 100×109 per liter, and evidence of a plasma leak based on changes in the hematocrit and pleural effusions) be met to support a diagnosis of dengue hemorrhagic fever — such that some patients with clinically severe disease were categorized inappropriately.32-34 With the recent revision of the World Health Organization (WHO) dengue classification scheme, patients are now classified as having either dengue or severe dengue.1,33,35 Patients who recover without major complications are classified as having dengue, whereas those who have any of the following conditions are designated as having severe dengue: plasma leakage resulting in shock, accumulation of serosal fluid sufficient to cause respiratory distress, or both; severe bleeding; and severe organ impairment. It is hoped that this system will prove more effective for triage and clinical management and will improve the quality of surveillance and epidemiologic data collected globally. Continued efforts through prospective multicenter studies are warranted to define the most appropriate classification scheme.
CLINICAL MANIFESTATIONS
After an incubation period of 3 to 7 days, symptoms start suddenly and follow three phases — an initial febrile phase, a critical phase around the time of defervescence, and a spontaneous recovery phase.
Febrile Phase
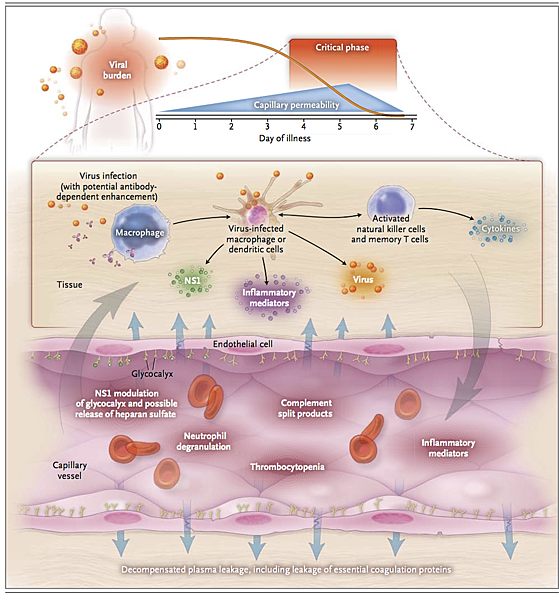
The initial phase is typically characterized by high temperature (≥38.5°C) accompanied by headache, vomiting, myalgia, and joint pain, sometimes with a transient macular rash. Children have high fever but are generally less symptomatic than adults during this phase of the illness. Mild hemorrhagic manifestations such as petechiae (Figure 3A)
and bruising, particularly at venipuncture sites (Figure 3B), and a palpable liver are commonly noted. Laboratory findings include mild-to-moderate thrombocytopenia and leukopenia, often with a moderate elevation of hepatic aminotransferase levels. This phase lasts for 3 to 7 days, after which most patients recover without complications.
Critical Phase
In a small proportion of patients, typically in children and young adults, a systemic vascular leak syndrome becomes apparent around the time of defervescence, evidenced by increasing hemoconcentration, hypoproteinemia, pleural effusions, and ascites. Initially, physiological compensatory mechanisms are up-regulated in an attempt to maintain adequate circulation to critical organs, resulting in narrowing of the pulse pressure when loss of plasma volume becomes critical. If the pulse pressure narrows to 20 mm Hg or less, accompanied by signs of peripheral vascular collapse, dengue shock syndrome is diagnosed and urgent, although careful, resuscitation is required. Systolic pressure may remain normal or even elevated at this time, and the patient may appear deceptively well, but once hypotension develops, systolic pressure decreases rapidly and irreversible shock and death may follow despite aggressive attempts at resuscitation. During the transition from the febrile to the critical phase, between days 4 and 7 of the illness, it is crucial for the clinician to be aware of warning signs that clinically significant vascular leakage may be developing in the patient. These signs of impending deterioration include persistent vomiting, increasingly severe abdominal pain, tender hepatomegaly, a high or increasing hematocrit level that is concurrent with a rapid decrease in the platelet count, serosal effusions, mucosal bleeding, and lethargy or restlessness.
Hemorrhagic manifestations are most common during this critical period. In children, clinically significant bleeding occurs only rarely, usually in association with profound and prolonged shock. However, major skin bleeding, mucosal bleeding (gastrointestinal or vaginal), or both may occur in adults with no obvious precipitating factors and only minor plasma leakage (Figure 3C).36 Moderate-to-severe thrombocytopenia is common, with nadir platelet counts below 20×109 per liter often observed during the critical phase, followed by rapid improvement during the recovery phase. A transient increase in the activated partial-thromboplastin time and a decrease in fibrinogen levels are also frequently noted. However, the coagulation profile is not typical of disseminated intravascular coagulation, and the underlying mechanisms remain unclear.37-39 Infrequently, other severe manifestations, including liver failure, myocarditis, and encephalopathy, occur, often with minimal associated plasma leakage.
Recovery Phase
The altered vascular permeability is short-lived, reverting spontaneously to a normal level after approximately 48 to 72 hours, and is concurrent with rapid improvement in the patient's symptoms. A second rash may appear during the recovery phase, ranging from a mild maculopapular rash to a severe, itchy lesion suggesting leukocytoclastic vasculitis that resolves with desquamation over a period of 1 to 2 weeks (Figure 3D). Adults may have profound fatigue for several weeks after recovery.
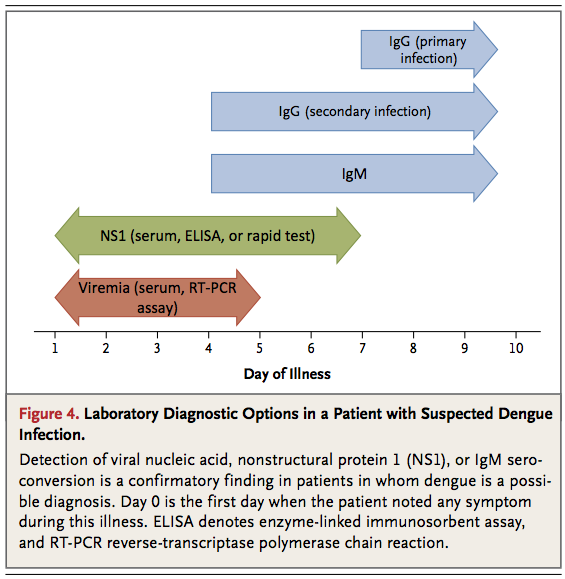
DIAGNOSTIC TESTS
Laboratory diagnosis of dengue is established directly by detection of viral components in serum or indirectly by serologic means. The sensitivity of each approach is influenced by the duration of the patient's illness (Figure 4)
During the febrile phase, detection of viral nucleic acid in serum by means of reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay or detection of the virus-expressed soluble nonstructural protein 1 (NS1) by means of enzyme-linked immunosorbent assay (ELISA) or the lateral-flow rapid test (not currently available in the United States) is sufficient for a confirmatory diagnosis. For primary infections in persons who have not been infected previously (which is typical in the case of most travelers), the diagnostic sensitivity of NS1 detection in the febrile phase can exceed 90%, and antigenemia may persist for several days after the resolution of fever. 40-42 The sensitivity of NS1 detection in the febrile phase is lower in secondary infections (60 to 80%), reflecting an anamnestic serologic response due to a previous dengue virus or related flavivirus infection.43
Serologic diagnosis of dengue relies on the detection of high levels of serum IgM that bind dengue virus antigens in an ELISA or a lateral-flow rapid test; IgM can be detected as early as 4 days after the onset of fever. IgM seroconversion between paired samples is considered a confirmatory finding, whereas detection of IgM in a single specimen obtained from a patient with a clinical syndrome that is consistent with dengue is widely used to establish a presumptive diagnosis. Commercially available IgM tests with acceptable performance characteristics have recently been identified.44 Serologic diagnosis of dengue can be confounded if the patient has very recently been infected or vaccinated with an antigenically related flavivirus (e.g., a virus associated with yellow fever or Japanese encephalitis). In addition, patients with secondary infections mount rapid anamnestic antibody responses in which dengue virus–reactive IgG may predominate over IgM. In clinical settings where methods of molecular detection (e.g., RT-PCR) are not available, investigation for elevated levels of dengue virus–reactive IgM or soluble NS1 in serum is a pragmatic diagnostic approach in a patient in whom dengue is suspected.43,45
MANAGEMENT
Currently, no effective antiviral agents to treat dengue infection are available, and treatment remains supportive, with particular emphasis on careful fluid management. 1 Patients who have no complications and are able to tolerate oral fluids may remain at home with instructions to return to the hospital immediately if bleeding or warning signs suggestive of vascular leakage develop. However, our practice is to evaluate these patients daily in a medical clinic with a complete blood count to monitor hematocrit and platelet values.
Development of any warning sign indicates the need for hospitalization and close observation, with judicious use of parenteral fluids in patients with inadequate oral intake or a rapidly increasing hematocrit. If the condition progresses to the dengue shock syndrome, prompt fluid resuscitation to restore plasma volume is imperative, followed by ongoing fluid therapy to support the circulation at a level just sufficient to maintain critical organ perfusion. Isotonic crystalloid solutions should be used, and isotonic colloid solutions should be reserved for patients presenting with profound shock or those who do not have a response to initial crystalloid therapy.46 To limit the risk of the development of fluid overload, parenteral fluid therapy should be kept to the minimum required to maintain cardiovascular stability until permeability reverts to a normal level.
Blood transfusion can be lifesaving for patients with severe bleeding that compromises cardiovascular function, but it should be undertaken with care because of the risk of fluid overload.Platelet concentrates, fresh-frozen plasma, and cryoprecipitate may also be needed depending on the coagulation profile. However, at present, there is no evidence that prophylactic platelet transfusions are of any value in patients who do not have clinically significant bleeding, even when thrombocytopenia is profound.47,48 The use of prophylactic platelet transfusions is increasing in countries where dengue is endemic, but given the associated clinical risks and the financial costs, controlled trials need to be performed before this becomes established as the standard of care. In patients with severe dengue infection, adjuvant therapy, including vasopressor and inotropic therapies, renal-replacement therapy, and further treatment of organ impairment, may be necessary.
The establishment of a therapeutic pipeline and the design of randomized, controlled trials of drugs targeting the virus or the immune response are recent developments. Recent trials have assessed chloroquine,49 oral prednisolone (A Randomized, Placebo-Controlled, Partially Blinded [Drug versus Placebo] Trial of Early Corticosteroid Therapy in Vietnamese Children and Young Adults with suspected Dengue Infection; Current Controlled Trials number, ISRCTN39575233), and balapiravir (A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of the Dengue Virus Polymerase Inhibitor [Balapiravir] in Male Patients with Confirmed Dengue Virus Infection; ClinicalTrials.gov number, NCT01096576), and further trials of statins and other antiviral drugs are planned. Currently, there is no evidence in favor of the use of any specific therapeutic agent for dengue.
EFFECTS ON HEALTH CARE SYSTEMS
Dengue imposes major demands on health care systems. Although severe dengue occurs in only a small proportion of dengue infections, early identification of high-risk patients is difficult and patients with uncomplicated infections are frequently hospitalized for observation. Rapid and effective triage by experienced personnel at the primary health care level, efficient and affordable transportation systems to facilitate daily clinical assessment, and public education campaigns to increase awareness of the disease all help to reduce unnecessary admissions. Among hospitalized patients, meticulous attention to detail is necessary to limit iatrogenic complications, including fluid overload. Ideally, patients with severe dengue infection should be treated in dedicated high-dependency units where frequent clinical observations by experienced staff with immediate access to repeated hematocrit measurements can ensure that fluid therapy is carefully titrated as needed. In such circumstances, mortality of less than 1% is achievable among patients with shock, and the need for ventilatory support and intensive care is minimized. Improvements in the early diagnosis and risk prediction of severe disease are urgently needed, especially in areas with a high case burden, where appropriate allocation of limited resources is crucial to the outcome. Ongoing research aims to refine the WHO 2009 classification scheme, particularly with regard to warning signs for the development of severe disease.
NEW APPROACHES TO TARGETING THE VECTOR
New vector-control approaches include the release of genetically modified male mosquitoes that sterilize the wild-type female population, thereby reducing egg output and the population size of the next generation that would be available for potential transmission of the dengue virus.50 An alternative strategy involves embryonic introduction of strains of the obligate intracellular bacterium wolbachia into A. aegypti. Strikingly, wolbachia-infected A. aegypti are partially resistant to dengue virus infection51,52 and can invade natural A. aegypti populations,51,53 suggesting the possibility of induction of widespread biologic resistance to dengue viruses in A. aegypti populations.
VACCINES
The leading dengue vaccine candidate, ChimeriVax (Sanofi Pasteur), is a tetravalent formulation of attenuated yellow fever 17D vaccine strains expressing the dengue virus prM and E proteins.54 It has been difficult to develop a vaccine for dengue that is safe and elicits balanced neutralizing antibody responses to all four serotypes. However, in the past 5 years, remarkable progress has been made, and multicenter phase 2–3 clinical trials that are designed to determine the efficacy of this three-dose vaccine are under way. Data on immunologic correlates of immunity are lacking. Long-term follow-up of vaccinees will be essential to understand whether waning vaccine-elicited immunity predisposes recipients to more severe outcomes on subsequent natural infection. Other candidates in early phases of clinical development include vaccines containing live attenuated dengue viruses and recombinant subunit vaccines.55
FUTURE DIRECTIONS
The field of dengue research has been invigorated over the past decade, fueled by the growing recognition of the burden of disease coupled with the prospect of a dengue vaccine. However, no vaccine can be an immediate global panacea, and efforts to improve treatment through application of existing best practices in triage and fluid management, along with efforts to develop new antiviral or other therapeutic drugs, must continue. Similarly, innovative approaches to preventing transmission of the virus, such as through modification of mosquito populations, should be fostered. An improved understanding of the current epidemiology of the disease and the potential for its future spread would also assist policymakers in allocating resources to combat this global public health challenge.
Dr. Simmons reports that his institution receives consulting fees on his behalf from Unither Virology and Tibotec and grant support on his behalf from Hoffmann–La Roche. No other potential conflict of interest relevant to this article was reported.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 