70-07 節錄
Invasive candidiasis (IC) is an important infection in the intensive care unit (ICU) setting given its association with poor clinical outcomes. The epidemiology of IC is complex and, although incompletely elucidated, is characterized by considerable regional and temporal variability. Overall, there appears to be an increase in the incidence of IC and a change in dis- tribution of the causative Candida spp. Of particular concern is an increase in the proportion of episodes caused by Candida glabrata, which is associated with reduced susceptibility to azole antifungal agents.
The management of IC has been aided by the availability of several new antifungal agents. In particular, given their broad spectrum of activity and low toxicity, the use of echinocandins as first-line therapy is increasing, especially in settings where fluconazole-resistant Candida spp. are prevalent. Fluconazole remains a reliable agent where an azole-susceptible pathogen is confirmed or in settings where resistance is uncommon. Lipid formulations of amphotericin B are now generally reserved as second-line or salvage therapy. Voriconazole and posaconazole currently enjoy limited use for IC in the ICU setting.
Although the poor outcomes associated with IC are, in part, related to the severity of underlying host factors, it is clear that optimization of treatment- related factors is also important. In particular, the speed of initiation of antifungal therapy and the achievement of pharmacodynamic parameters both influence outcomes. The most difficult challenge is early initiation of an effective antifungal drug, given the slow turnaround time and lack of sensi- tivity of conventional culture-based diagnostic techniques. New approaches, such as non-culture-based assays and/or clinical risk-predictive models are required to better target prophylactic, pre-emptive and empirical antifungal strategies.
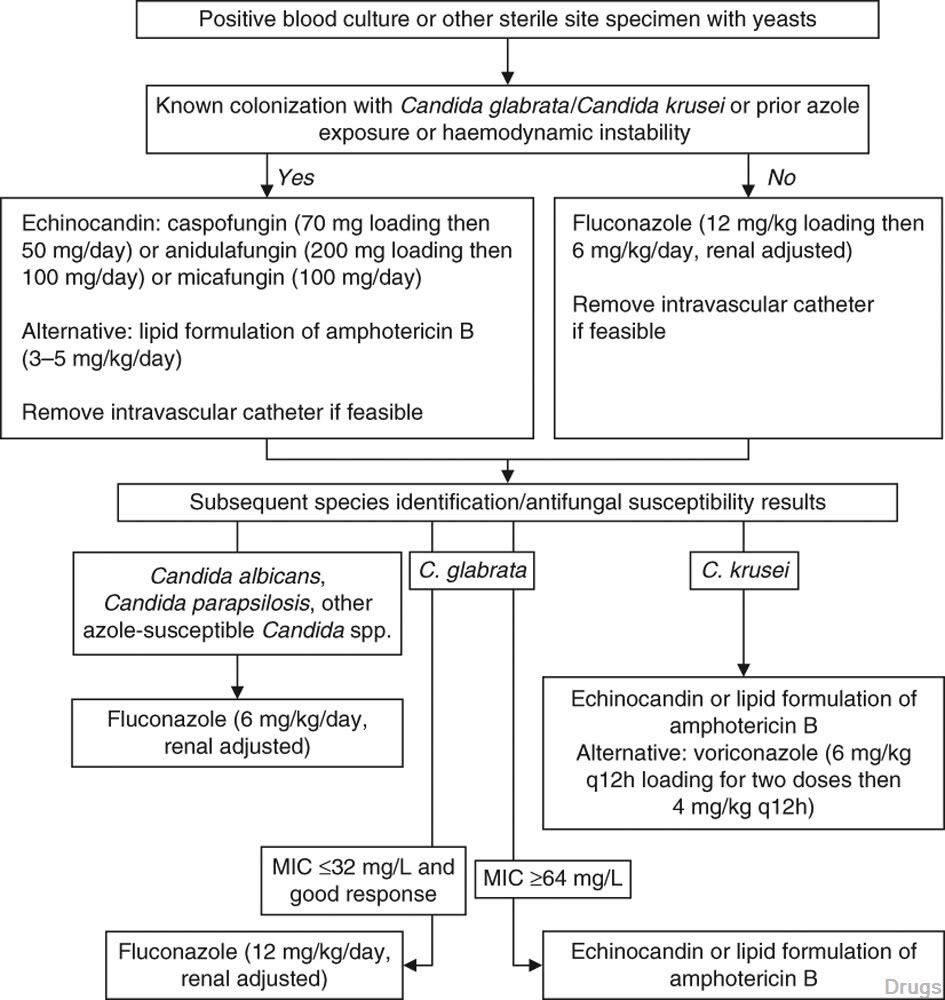
Table I. Randomized clinical trials in invasive candidiasis (IC) among predominantly non-neutropenic patients
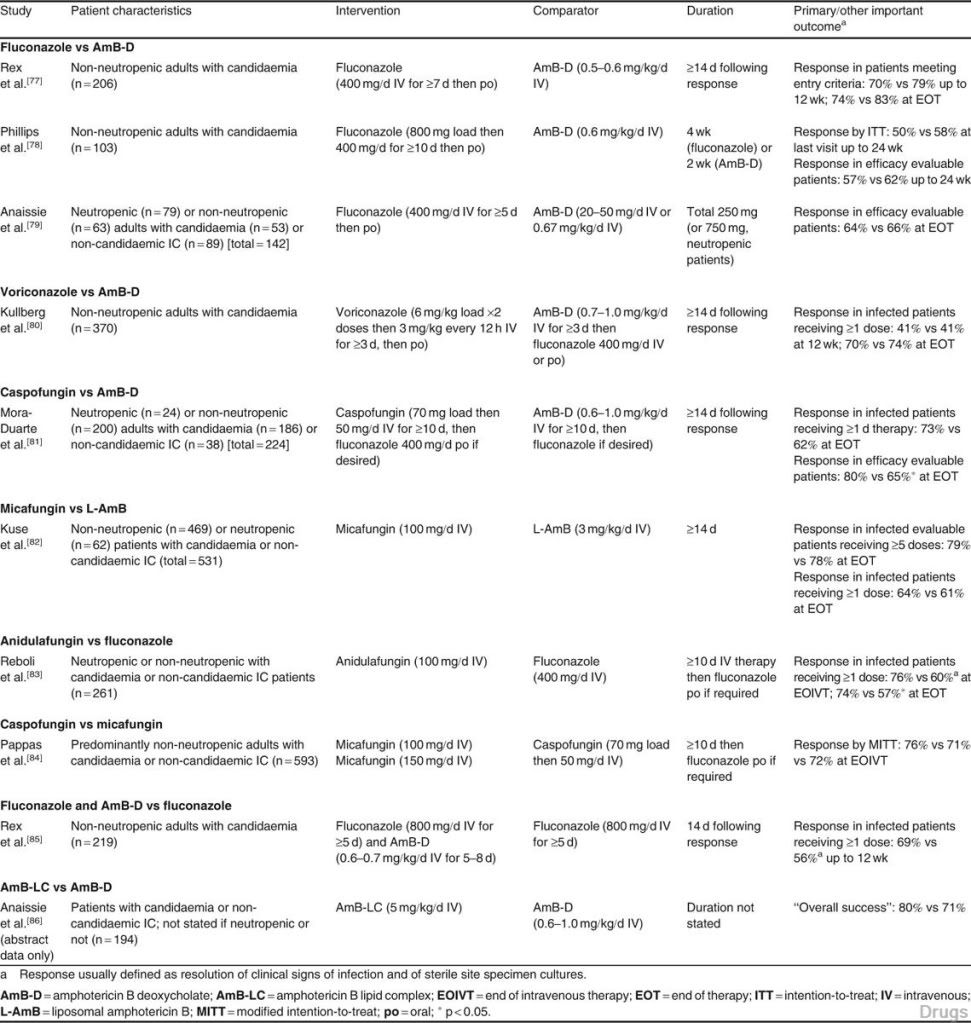
Fig. 1. Algorithm for the management of invasive candidiasis in the intensive care unit setting. Total duration is 14 days following clinical and microbiological resolution. Where clinical response is suboptimal and/or blood cultures remain positive, change to echinocandin or lipid formulation of amphotericin B whilst assessing for antifungal resistance, intravascular/deep infective focus or effects of underlying immuno- suppression. MIC = minimum inhibitory concentration; q12h = every 12 hours.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 