70-07 節錄
This review describes the current status of antiplatelet therapy in preven- tion of cardiovascular events of an atherothrombotic nature. The efficacy of aspirin clearly outweighs bleeding risk in secondary prevention, with the re- levant exception of patients with peripheral arterial disease (PAD). In trials of primary prevention, aspirin has a limited advantage, which is challenged by the risk of major bleeding. A typical example is primary prevention in type 2 diabetes mellitus, in which a number of trials and a recent meta-analysis have confirmed these limitations.
In various settings, clopidogrel has been shown to be marginally more effective than aspirin. Despite a non-negligible bleeding risk, the combination of aspirin-clopidogrel has provided satisfactory results in conditions at high thrombotic risk but rather disappointing results in the long-term treatment of chronic stable cardiovascular disease. The combination of aspirin-dipyridamole was shown to be superior to aspirin alone and equivalent to clopidogrel alone for secondary prevention in cerebrovascular patients.
Limitations in the efficacy of antiplatelet agents are partly inherent in their mechanism of action and should not be considered simply as ‘treatment failures’. Among other factors, individual variability of response to anti- platelet drugs also plays a meaningful role. Variability of response and ‘re- sistance’ may result from drug interactions, baseline and residual platelet hyperactivity, increased platelet turnover, pharmacogenetic factors and others. Poor biological response to aspirin and/or clopidogrel is also frequent in clinical settings such as diabetes, obesity and acute coronary syndromes. The correlation between biological resistance and impaired clinical efficacy of aspirin, and especially clopidogrel, is currently accepted, although with lim- itations due to the different methods used to assess platelet response. Indeed, the concept of individual ‘tailoring’ of antiplatelet regimens on the basis of previous laboratory or ‘point of care’ platelet function tests has been vali- dated in a number of recent trials.
The search for and validation of new antiplatelet agents with already known, or totally new, mechanisms of action have also been undertaken with increasing eagerness. Among new adenosine diphosphate receptor antag- onists, prasugrel is already registered, and ticagrelor and cangrelor are being developed. New mechanisms being explored are blockade of thrombin- induced platelet aggregation (vorapaxar [SCH 530398]), and inhibition of collagen and ristocetin-mediated platelet functions (DZ-697b).
Reappraisal of the neglected class of direct thromboxane A2 antagonists was followed with less interest. Besides blocking the effects of thromboxane produced from platelets, drugs of this class (such as terutroban sodium and picotamide) may also protect cells from thromboxane produced by sources other than platelets, and some of them may preserve or enhance prostacyclin production. Terutroban is presently being tested in PAD and stroke preven- tion. Picotamide, marketed in Italy, was shown to reduce cardiovascular events and mortality in studies of PAD patients with diabetes. The results available with thromboxane inhibitors are particularly interesting because they are being obtained in conditions, such as type 2 diabetes and PAD, which are known to be refractory to aspirin.
Table I. Clinical trial acronyms
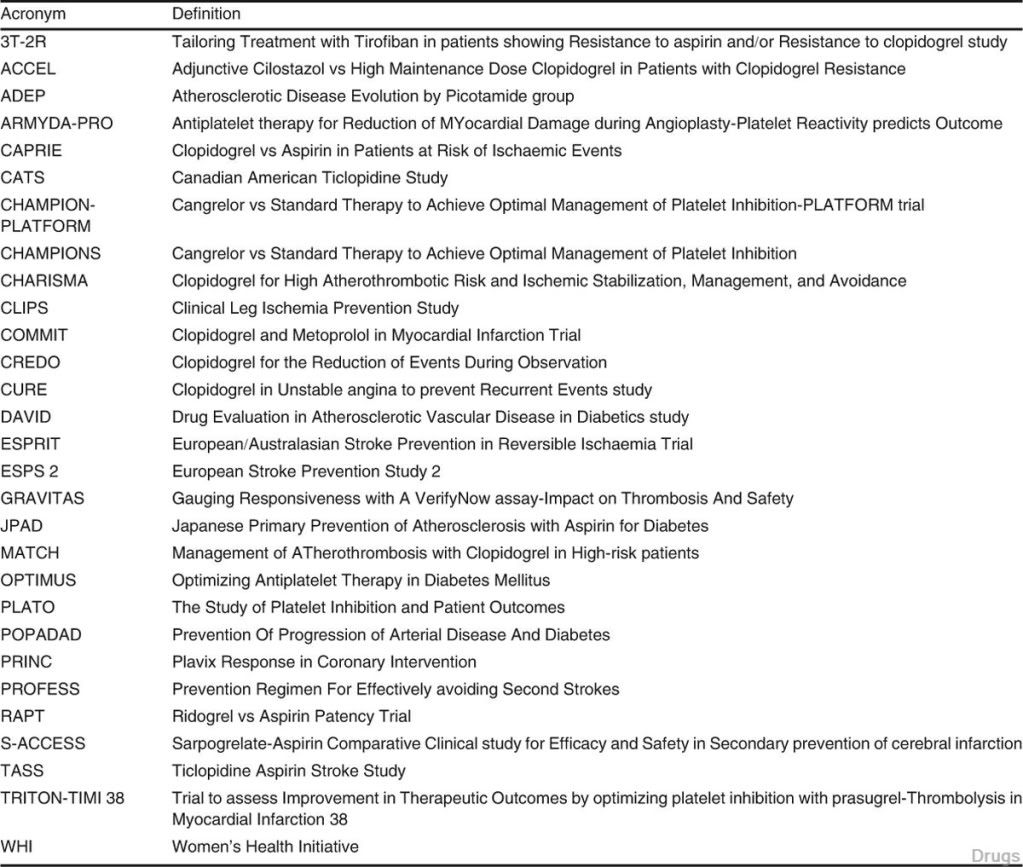
Fig. 1. Catabolism of platelet membrane arachidonic acid. Cyclo- oxygenase (COX)-1 or, in inflammatory conditions, the inducible COX-2 catalyse endoperoxide (PGH2) formation. In turn, thrombo- xane synthase induces formation of thromboxane A2 (TXA2; see text section 4.1) which is excreted in the form of two final metabolites as thromoxane B2 (TXB2). A similar enzymatic chain occurs in endo- thelial cells leading to formation of prostacyclin (PGI2)
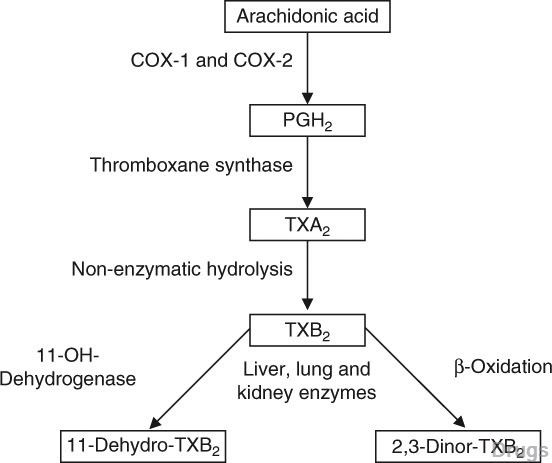
Table II. Main novel antiaggregating agents or adjuvant drugs





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 