Rolofylline,之前叫做MK-7418,是目前墨克開發中系列的一員(Anacetrapib、Taranabant、Deforolimus、Rolofylline、Odanacatib、Laropiprant),功能是用在急性心衰竭,在在PROTECT試驗中證實:靜注300mg/dL 的rolofylline能改善急性心衰
伴隨腎功不全者的心衰症狀,且使血肌酐水平下降。不過整體三期實驗在結果上並沒有想像中的理想,所以應該還會拖一陣子吧。
Preexisting chronic kidney disease and worsening renal function are common in patients hospitalized with acute heart failure and are associated with poor outcomes.1-5 Multiple factors are responsible for this association,3-5 including coexisting conditions, less use of effective therapies in patients with renal dysfunction than in patients without renal dysfunction, and inadequate treatment of volume overload because of a suboptimal response to diuretics or concern regarding diuretic toxicity.4,5
Adenosine has been implicated as an important intrarenal mediator of both worsening renal function and diuretic resistance.6,7 ATP hydrolysis releases free adenosine into the extracellular space, which in turn acts on adenosine A1 receptors in the afferent arterioles, reducing renal blood flow and the glomerular filtration rate (GFR) and stimulating the release of renal renin. In addition, A1-receptor activation enhances proximal tubular sodium reabsorption.6,7 In patients with heart failure, A1-receptor antagonists may preserve the GFR, enhance sodium excretion, and improve diuretic responsiveness.
Previous studies involving patients with heart failure have shown that coadministration of A1-receptor antagonists and loop diuretics enhances diuresis while maintaining or improving renal function.8-10 In the PROTECT (Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) pilot study, a dose-finding study,11 rolofylline at a dose of 10, 20, or 30 mg or placebo was administered daily for up to 3 days in patients with acute heart failure, underlying renal dysfunction, and volume overload. As compared with patients who received placebo, patients who received the 30-mg dose of rolofylline had greater relief of dyspnea and less worsening of renal function, with a trend toward fewer deaths or readmissions for heart or renal failure. The present phase 3 PROTECT trial was designed to confirm these findings.
METHODS
Study Design and Oversight
This randomized, double-blind trial compared rolofylline with placebo in patients hospitalized for acute heart failure at 173 sites in North America, Europe, Israel, and Argentina. The study, which was sponsored by NovaCardia, a subsidiary of Merck, was designed by an executive committee consisting of seven investigators and two Merck clinical scientists and was based largely on the design and results of the pilot study.11 The background, design, and results of the pilot study, as well as a summary of the design and protocol modifications that were made for the phase 3 study, are included in the Supplementary Appendix, available with the full text of this article at NEJM.org. The protocol was approved by the ethics committee at each participating center, and patients provided written informed consent.
The study was monitored by an independent data and safety monitoring committee, which was supported by an independent statistical center with a staff that was aware of the study-drug assignments (Statistics Collaborative). The only stopping rule for efficacy was a reduction in 180-day mortality crossing an O'Brien−Fleming boundary that preserved an overall (one-sided) false positive error rate of 0.005.13 Hospitalizations, central nervous system events, and deaths within 60 days after study-drug administration were adjudicated by an independent clinical events committee that was unaware of treatment assignments. The site investigators gathered the primary data, which was monitored by an independent contract research organization (Averion International, formerly Hesperion). Statistical analyses were performed by Averion with the use of SAS software, version 8.2 (SAS Institute), and these analyses were subsequently confirmed by Merck. The executive committee had full access to the final data set. The first author prepared the initial draft of the manuscript, which was revised on the basis of the comments of the other authors, who each approved the final version. All authors vouch for the accuracy and completeness of the reported data as well as the fidelity of the reported results to the trial protocol.
Study Patients
Eligibility criteria included persistent dyspnea at rest or with minimal activity, impaired renal function (an estimated creatinine clearance of 20 to 80 ml per minute with the use of the Cockcroft−Gault equation), a brain natriuretic peptide level of 500 pg per milliliter or more or an N-terminal pro-brain natriuretic peptide level of 2000 pg per milliliter or more, ongoing intravenous loop-diuretic therapy, and enrollment within 24 hours after admission. Other inclusion and exclusion criteria have been described previously12 and are included in the Supplementary Appendix.
Since adenosine A1−receptor antagonists may lower the threshold for seizures in predisposed patients, those with a history of seizures or predisposing factors for seizures were excluded.12 Patients with a more distant history of conditions or factors associated with a lower seizure risk were pretreated with 1 mg of oral lorazepam or clonazepam 30 minutes before administration of the study drug. The criteria used to define a low seizure risk are available in the Supplementary Appendix.
Study Procedures
Thirty milligrams (0.5 mg per milliliter) of rolofylline (NovaCardia) or matching placebo was administered as a 4-hour intravenous infusion daily for up to 3 days in a double-blind manner according to a computer-generated randomization scheme (in a 2:1 ratio of rolofylline to placebo), assigned through a central randomization system.
Symptoms and signs of heart failure were evaluated before the initial administration of the study drug, daily through discharge or day 6, and on days 7 and 14. Patients reported symptoms of change in their breathing and their general well-being with the use of a 7-point Likert scale of change relative to baseline (ranging from −3 to 3, with higher scores indicating greater improvement). Moderately or markedly improved dyspnea was defined as a score of either 2 or 3 on this scale. Measurements of the serum creatinine level were recorded at the same time points.14,15 Worsening heart failure in the period from study-drug initiation through day 7 was reported on the basis of worsening signs or symptoms leading to intensification of therapy.
Study End Points
The primary end point was treatment success, treatment failure, or no change in the patient's condition. Success was defined as patient-reported moderate or marked improvement in dyspnea both 24 and 48 hours after administration of the study drug, in the absence of any criterion for failure. Failure was defined as the occurrence of any of the following: death or readmission for heart failure through day 7, worsening symptoms and signs of heart failure occurring more than 24 hours after the initiation of the study drug requiring intervention by day 7 or discharge (if earlier), or persistent worsening renal function, defined as an increase in the serum creatinine level of 0.3 mg per deciliter (26.5 μmol per liter) or more from randomization to day 7, confirmed at day 14, or the initiation of hemofiltration or dialysis during the period from initiation of the study drug through day 7. Patients were classified as having unchanged treatment status if they met neither the criteria for treatment success nor the criteria for treatment failure.
Two secondary outcomes were prespecified: death from any cause or rehospitalization for cardiovascular or renal causes through day 60 and the proportion of patients with persistent renal impairment, defined as an increase in the serum creatinine level of 0.3 mg per deciliter or more by day 7, confirmed at day 14; the initiation of hemofiltration or dialysis through day 7; or death by day 7.
Adverse events were recorded through day 7, and serious adverse events were recorded through day 14; they were classified according to the Medical Dictionary for Regulatory Activities. Safety was evaluated on the basis of the incidence of adverse events and laboratory abnormalities. The seriousness of adverse events and their relatedness to the study drug were determined by the investigators. Patients or family members were contacted by telephone to identify deaths and readmissions up to day 60 and to assess vital status at day 180. Patients who were hospitalized were asked about the reason for readmission.
Statistical Analysis
PROTECT was initially planned as two identical 600-patient studies to be conducted simultaneously (PROTECT-1 and PROTECT-2; ClinicalTrials.gov numbers, NCT00328692 and NCT00354458, respectively). In this article, the combined trials are referred to as PROTECT. The first patient was enrolled in May 2007, and in December 2007, the protocol was amended to specify a combined analysis of the two studies and an increase in sample size from 1200 to 2000 patients to maintain 90% power with a more stringent definition of significance.
Efficacy end points were evaluated in the intention-to-treat population. The effectiveness of rolofylline with respect to the primary end point was evaluated in the combined studies at a significance level of 0.00125. The planned sample of 2000 patients provided approximately 90% power to detect a difference between a distribution of 25% failure, 34% no change, and 41% success in the rolofylline group and a distribution of 33% failure, 35% no change, and 32% success in the placebo group with the use of the Wilcoxon test.
If the primary end point was achieved, both secondary end points were to be tested at a nominal two-sided significance level of 0.05. The study design provided 95% power at the two-sided significance level of 0.05 to detect a hazard ratio of 0.74 for death or rehospitalization for cardiovascular or renal causes and a 33% relative reduction in the rate of persistent renal impairment with the use of a Cochran−Mantel−Haenszel test.
The study groups were compared with respect to the primary end point with the use of the van Elteren extension of the Wilcoxon test,16 stratified according to study and geographic region of enrollment (region 1 consisted of North America, Western Europe, and Israel, and region 2 consisted of Central Europe, Eastern Europe, and Argentina). The odds ratio and 95% confidence interval for the treatment effect were determined from an ordered logistic-regression (proportional-odds) model that included terms for the effects of treatment, study, and geographic region. Given the classification (−1 denoting failure, 0 no change, and 1 success), an odds ratio of less than 1.0 would favor active treatment.
For the end point of time to death from any cause or rehospitalization for cardiovascular or renal causes through day 60, the treatment groups were compared with the use of a Cox regression model, stratified according to study and geographic region. Cumulative event rates were calculated with the use of the Kaplan−Meier method. The proportion of subjects with persistent renal impairment was analyzed with the use of a Cochran−Mantel−Haenszel test, stratified according to study and geographic region.
For the primary end point and the two secondary end points, the treatment effect was evaluated across 11 prespecified baseline subgroups according to the study (PROTECT-1 or PROTECT-2), geographic region, sex, age (≤70 years or >70 years), race (white or other), ethnic group (Hispanic or Latino, or other), pretreatment or no pretreatment with a benzodiazepine, left ventricular ejection fraction (<40% or ≥40%), baseline serum creatinine level (less than the median or greater than or equal to the median), severity of heart failure (New York Heart Association class I or II, III, or IV), and baseline creatinine clearance (<30, 30 to <60, 60 to <80, or ≥80 ml per minute).
RESULTS
Patients
Between May 2007 and January 2009, a total of 2033 patients were randomly assigned to a study drug (1356 to rolofylline and 677 to placebo). Of these patients, 21 who were assigned to the rolofylline group and 10 who were assigned to the placebo group did not receive the study drug and were excluded from safety analyses. Randomization of the patients, treatment, and outcomes are shown in Figure 1 in the Supplementary Appendix. Only 1 patient was lost to follow-up at the 60-day assessment; vital status at 180 days could not be ascertained for 5 patients.
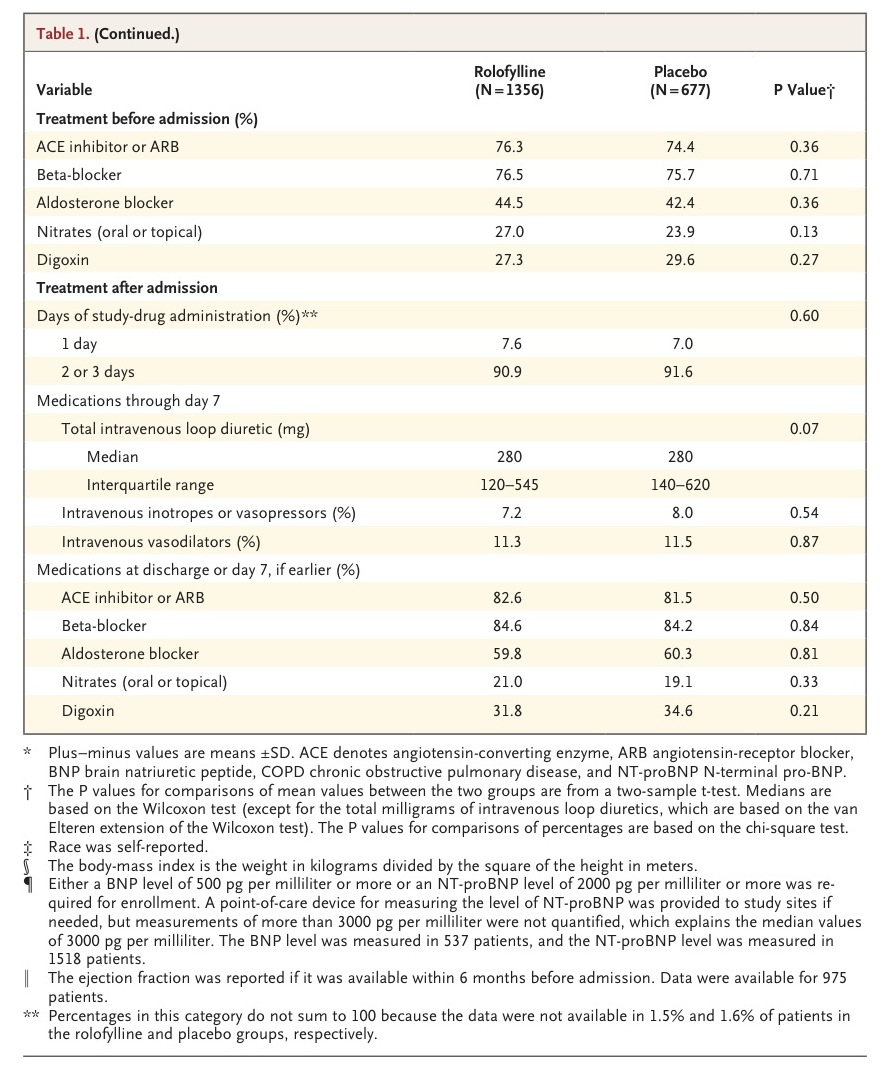
Important demographic, clinical, and treatment characteristics of the patients are shown in table1
The study groups were well matched with respect to all baseline variables. Most patients had chronic heart failure before admission, ischemic heart disease, and one or more additional coexisting conditions. The mean creatinine clearance was 51 ml per minute, and levels of natriuretic peptides were substantially elevated.
More than 90% of patients received two or three doses of the study drug. The median doses of intravenous loop diuretics (in furosemide dose equivalents) administered from randomization through day 7 or discharge, if earlier, were 280 mg (interquartile range, 120 to 545) in the rolofylline group and 280 mg (interquartile range, 140 to 620) in the placebo group (P=0.07). The highest quartile of diuretic doses was substantially higher in the placebo group than in the rolofylline group. Weight loss during the first 4 days was greater with the use of rolofylline (3.0 vs. 2.6 kg, P=0.005). Additional intravenous vasoactive agents were administered before day 7 in 17% of the patients in the rolofylline group and 16% of the patients in the placebo group.
Primary Efficacy End Points
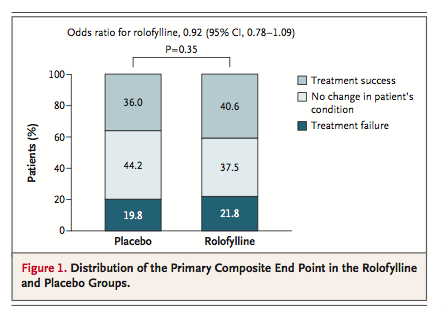
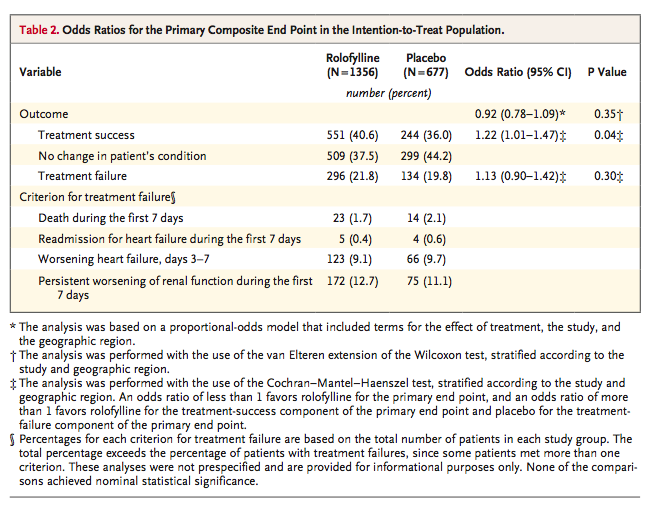
Rolofylline, as compared with placebo, was not beneficial with respect to the primary end point (the proportions of patients in whom treatment was successful, treatment failed, or there was no change in the patient's condition), yielding an odds ratio of 0.92 (95% confidence interval [CI], 0.78 to 1.09;P=0.35)
More patients in the rolofylline group than in the placebo group met the criteria for treatment success (40.6% vs. 36.0%; odds ratio, 1.22; 95% CI, 1.01 to 1.47; P=0.04). However, the proportion of treatment failures was also higher in the rolofylline group than in the placebo group (21.8% vs. 19.8%; odds ratio, 1.13; 95% CI, 0.90 to 1.42; P=0.30), reflecting a numerical excess of patients who met the criteria for worsening renal function (12.7% vs. 11.1%, P=0.31). Other criteria for treatment failure were similar in the rolofylline and placebo groups, including early death (in 1.7% and 2.1% of patients in the two groups, respectively), early worsening heart failure (9.1% and 9.7%), and rates of early readmission for heart failure (0.4% and 0.6%). There was no clear evidence of heterogeneity in outcome among the prespecified subgroups or in post hoc analyses involving patients with or without diabetes or diuretic doses above or below the median (Figure 2A in the Supplementary Appendix).
Secondary Efficacy End Points
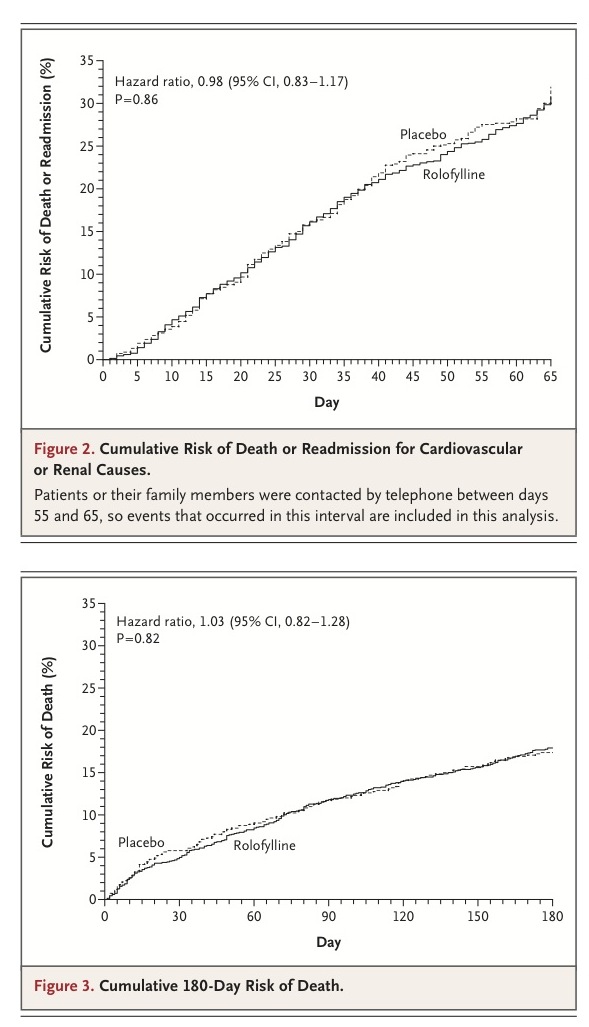
Through day 60, a total of 386 of 1356 patients assigned to rolofylline (Kaplan–Meier estimate, 30.7%; 95% CI, 27.8 to 33.6) as compared with 195 of 677 patients assigned to placebo (Kaplan–Meier estimate, 31.9%; 95% CI, 27.4 to 36.4) died or were readmitted for cardiovascular or renal causes (hazard ratio, 0.98; 95% CI, 0.83 to 1.17; P=0.86)
Persistent renal impairment occurred in 15.0% of the patients in the rolofylline group as compared with 13.7% of the patients in the placebo group (odds ratio, 1.11; 95% CI, 0.85 to 1.46; P=0.44). These results were generally consistent across subgroups (Figure 2 in the Supplementary Appendix). Rates of death over a period of 180 days were similar (17.9% in the rolofylline group and 17.4% in the placebo group; hazard ratio, 1.03; 95% CI, 0.82 to 1.28; P=0.82)
Safety
At least one adverse event was reported in 840 of 1336 patients treated with rolofylline (62.9%) and in 409 of 666 patients who received placebo (61.4%). In the rolofylline group, 185 patients had serious adverse events (13.8%) as compared with 98 patients in the placebo group (14.7%). These serious adverse events led to discontinuation of the study drug in 1.5% of the patients in each group; they were associated with a fatal outcome in 3.7% of the patients in the rolofylline group and 5.3% of the patients in the placebo group. Most serious adverse events were cardiac events; these were reported in 96 patients who received rolofylline (7.2%) and 60 patients who received placebo (9.0%). The rates of the two most frequent serious cardiac events, worsening heart failure and ventricular tachycardia, both tended to be lower among patients who received rolofylline (P=0.04 and P=0.10, respectively).
Imbalances were observed with respect to the investigator-reported neurologic adverse events. By day 14, a total of 11 patients in the rolofylline group (0.8%), but no patients in the placebo group, had seizures (risk difference, 0.8 percentage points; 95% CI, 0.3 to 1.5; P=0.02), of whom only 1 had received pretreatment with a benzodiazepine. Fifteen patients who received rolofylline (1.1%) and 2 patients who received placebo (0.3%) had strokes (risk difference, 0.8 percentage points; 95% CI, 0.0 to 1.6; P=0.06). There was no particular pattern of stroke events in the rolofylline group with regard to the stroke type (5 were hemorrhagic, 6 were ischemic, 3 were embolic, and 1 was a spinal cord infarction) or the temporal relationship to administration of the study drug (10 occurred within 6 days after the last dose and 5 occurred ≥7 days after the last dose) (Table 1 in the Supplementary Appendix).
DISCUSSION
The clinical and economic burdens of heart failure have been well described.17 Treatment advances over the past 25 years have improved symptoms and quality of life and increased survival among patients with chronic heart failure.18 In contrast, although approximately one third of patients with acute heart failure die or are readmitted within 3 months after discharge,19-21 new therapies have consistently failed to improve their outcomes.20 Patients with underlying chronic kidney disease or worsening renal function are at particularly high risk.1,2,4,5,22,23
As a result, there has been considerable interest in A1-receptor antagonists, which have enhanced the response to diuretics in patients with heart failure, usually without further deterioration of renal function.7-10,23-25 With the use of a protocol that was similar to that in the present trial, the PROTECT pilot study randomly assigned 301 patients with acute heart failure to placebo or to 10-, 20-, or 30-mg doses of rolofylline.11 In the pilot trial, the group of patients who received 30 mg of rolofylline were more likely than those who received placebo to have improvement in dyspnea on days 2 and 3 (59.4% of patients vs. 41.3%) and were less likely to have persistent renal impairment (8.0% vs. 18.2%), with a trend toward a lower 60-day rate of death or readmission for cardiovascular or renal causes (19% vs. 34%; hazard ratio, 0.55; 95% CI, 0.28 to 1.04; P=0.06).
However, despite similarities in study design, entry criteria, and dose of rolofylline, the main trial reported here did not replicate the findings of the pilot trial. Several reasons for this discordance warrant consideration. The protocol underwent several revisions. First, one of the inclusion criteria for the current trial, but not for the pilot trial, was an increased level of brain natriuretic peptide or N-terminal pro-brain natriuretic peptide; nonetheless, nearly 80% of the patients in the pilot trial would have qualified for the present trial. Second, the criterion for successful treatment was changed from a physician-directed switch from intravenous to oral diuretics to patient-reported improvement in symptoms. Nonetheless, rolofylline was associated with a significantly higher rate of success than placebo. Finally, on the basis of the data from the pilot study and regulatory input, the definition of persistent renal impairment was changed from an increase in the serum creatinine level of 0.3 mg per deciliter or more from baseline to discharge or day 7 to an increase at both day 7 and day 14. The neutral results of PROTECT primarily reflect this lack of a favorable effect on the serum creatinine level.
Perhaps the most likely explanation for the differences between the findings in the two studies is the large uncertainty about the results of the pilot study because of the small treatment groups and the dependence on these findings in designing critical elements of the main trial, including the selection of a single dose, entry criteria, and end points.11 The post hoc selection of the best of three dose groups from a pilot trial with multiple small treatment groups carries the inherent risk that an apparent superiority may be the play of chance and that these findings may not be replicable in a more definitive, larger study, as happened in the current trial. These findings should encourage the conduct of larger phase 2 studies with more robust, clinically relevant end points.
A total of 0.8% of the patients who received rolofylline (as compared with none who received placebo) had seizures, a recognized risk of agents with A1 receptor−antagonist activity because of their tendency to lower the threshold for seizures.26,27 The excess of strokes in the rolofylline group was unanticipated, is difficult to interpret, and is not clearly explained by the pharmacologic characteristics of the drug.
In conclusion, the primary and secondary end points were not achieved with rolofylline in the current trial. The current trial and previous unsuccessful trials of treatment for acute heart failure highlight the complexity and heterogeneity of this clinical syndrome and the need for new, more effective therapeutic approaches.
Supported by NovaCardia, a subsidiary of Merck.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 