
Welcome and Introduction
Paolo Raggi, MD: Hello, I'm Dr. Paolo Raggi. I'm a Professor of Medicine at Emory University School of Medicine in Atlanta, Georgia. It is my pleasure to welcome you to this Medscape CME/CE activity entitled Are We Doing More Harm Than Good? New Perspectives on Calcium Balance in Chronic Kidney Disease (CKD). I'm honored to introduce my friend and colleague, Dr. David Bushinsky, Professor and Chief of Nephrology at the University of Rochester Medical Center in Rochester, New York. Welcome to today's program, David.
David Bushinsky, MD: Hello. It's a pleasure to be here.

Slide 2. Dr. Raggi: The goal of today's discussion is to review the latest advances in the pathophysiology of CKD and the effect of treatment on the potential for calcium retention in the body. We will also be translating the mathematical concepts describing calcium balance and homeostasis into practical clinical hints. Dr. Bushinsky: Yes. Dr. Raggi: So, David, let's start with an obvious question. How does the human body acquire and dispose of calcium?
Calcium Balance
Slide 3.
Dr. Bushinsky: Paolo, as you well know, there's calcium in many foods, especially dairy products. As we ingest calcium, we absorb much of that calcium into our body. That absorption is aided by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the activated form of vitamin D. So when we eat calcium, it's absorbed into our body. If we're children and growing or if we're a woman who is pregnant, we would need more calcium on a daily basis to grow those infants' bones or the fetus's bones or to grow our own bones. After we reach full adult height, we no longer need that additional calcium. And any calcium that's absorbed and not needed is excreted by the kidney.
Dr. Raggi: And that would be in a normal --
Dr. Bushinsky: Be in an individual with normal kidney function.
On a daily basis, I make bone and break down bone, and that bone breakdown and accrual is about 10% of our total body bone on a yearly basis. So, it's an active dynamic process, but the principal thing to remember is that we are in neutral calcium balance. We absorb calcium and we excrete exactly as much calcium as we absorb.
Dr. Raggi: And so, as our kidneys fail, what's happening to the normal homeostasis of calcium and phosphorus?
Slide 4.
Dr. Bushinsky: That's a great question. As our kidneys fail, the body is striving to maintain the level of serum calcium. As you know -- and you're a cardiologist -- if the calcium goes too high or too low, the heart doesn't function properly nor do many other organs. So, the body strives to maintain a normal calcium balance. How does it do this? It changes the hormonal milieu. It lowers the vitamin D level and increases the level of parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). And with those changes, serum calcium concentration is maintained at a beautifully normal level until the kidneys almost fail, and we see the calcium start to go down just a little bit.
Dr. Raggi: So, do we see any abnormal calcium levels and does it mean anything? What I'm trying to lead to is, are we too tied to a serum level of calcium and phosphorus for us to decide if something is abnormal in the homeostatic environment?
Dr. Bushinsky: Right. That's a good question. The body strives to maintain that absolute normal level of serum calcium, but that doesn't tell us anything about how much calcium is retained in the body. A perfect example of this is an elderly woman with osteoporosis. She has far less calcium in her body at age 75 than she did at age 30, yet her serum calcium concentration is absolutely normal. So, the next important point is that the serum calcium level tells us nothing about how much calcium is contained in the bones or in the soft tissue.
Slide 5.
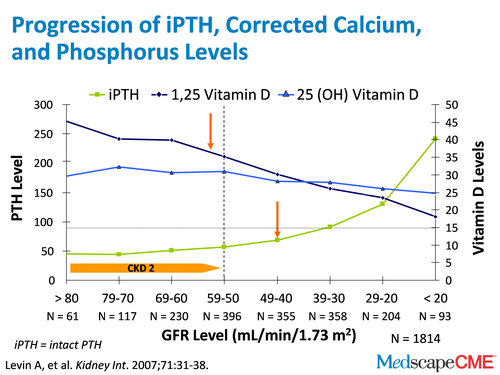
Dr. Raggi: So, if I understood correctly, which is something that you mentioned earlier, is that there are some changes both in vitamin D level, PTH, and changes with calcium and phosphorus in our body, as the kidneys fail?
Dr. Bushinsky: Right.
Dr. Raggi: When do we start detecting these changes?
Dr. Bushinsky: So a number of investigators have looked at this. In this study, it's amazing that at a glomerular filtration rate (GFR) of about 50 mL/min/1.73 m2, when we lose about half of our renal function, we start to see changes in PTH and 1,25 (OH)2D3. PTH is going up and 1,25 D is going down. But even earlier, we start to see changes in this new [clinical marker] called FGF-23. And we think it all starts with retention of phosphorus in our body. As the kidneys fail, we ingest phosphorus but we can't excrete the phosphorus quantitatively that's absorbed. And so, the body has to rid itself of phosphorus.
Slide 6.
So, FGF-23, which is a phosphaturic hormone in response to levels of phosphorus, begins to rise. 1,25 D begins to fall so we absorb less phosphorus and PTH rises, which also helps FGF-23 increase phosphorus excretion from the body. So these hormonal changes occur early, are quite significant, and their goal is to maintain normal serum levels of calcium and phosphorus, but they don't really have any role in phosphorus and calcium retention in the body.
Dr. Raggi: Interesting. So, at some point, there will be too high a PTH level, and too low a vitamin D level.
Dr. Bushinsky: Right.
Dr. Raggi: What happens at that point? What do we call this stage? You mentioned that I'm a cardiologist, so educate me on the basics here.
Secondary Hyperparathyroidism
Slide 7.
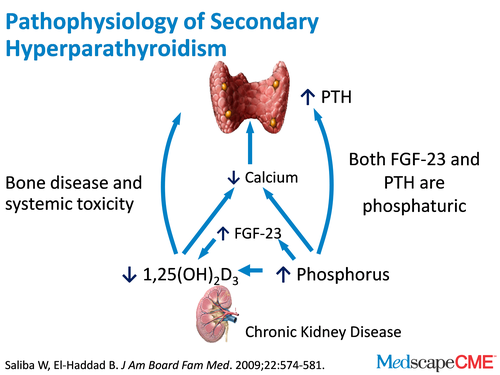
Dr. Bushinsky: A very knowledgeable cardiologist [laughter]. So we develop a syndrome called secondary hyperparathyroidism and secondary hyperparathyroidism is exactly what it says. The PTH level is elevated secondary to the hormonal changes we described before. When the kidneys fail, there is no longer that outlet of calcium from the body so any calcium that we absorb, we can't excrete. Several studies have shown that in CKD stages III and IV, urine calcium goes down to trivial levels, to 50 mg in CKD stages III and IV, and down to essentially 0 mg by the time patients are on dialysis.[1,2] So, we have a problem. We're absorbing calcium and have no way to excrete it. This is also compounded by the fact that if we don't have functioning kidneys and with secondary hyperparathyroidism, the high PTH is causing calcium to be released from bone. High PTH causes some bone formation, but bone reabsorption exceeds bone formation and calcium moves from the bone and intestine into the extracellular fluid space. You, as a cardiologist, know that if that calcium is moving out of bone into the vascular space, into the extracellular fluid space and it can't be excreted by the kidneys, where does it go?
Dr. Raggi: Absolutely. We definitely have a large discussion to conduct on vascular calcification, but before I get there, you mentioned something that I would like to go back to for a second. You mentioned calcium is absorbed by and through the intestine. So, this is very important. How much calcium can we absorb as we fail with our kidneys, or even without kidney failure?
Slide 8.
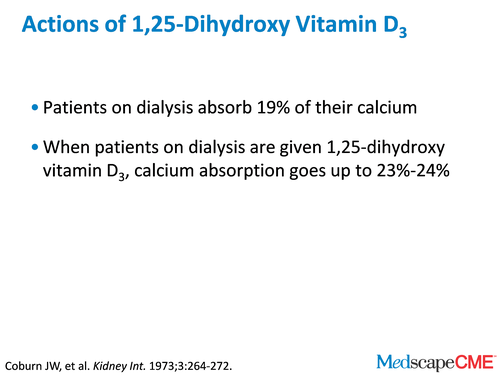
Dr. Bushinsky: Years ago, the late Jack Coburn did very sophisticated studies and demonstrated that baseline dialysis patients absorb about 19% of their calcium. But if they're given activated vitamin D, which in his case was 1,25 (OH)2D3, the absorption goes up to maybe 23% or 24%.
Dr. Raggi: That's important and definitely an element to be considered when we administer a lot of calcium-based binders or calcium supplements to our patients with renal failure. So, let's talk a little bit, if you don't mind, about the pathophysiology of vascular calcification. Is it a problem?
Dr. Bushinsky: Yes.
Dr. Raggi: How prevalent is it? And do you think it's a problem from the point of view of outcome on your patients?
Slide 9.

Dr. Bushinsky: So obviously, as a cardiologist and as someone who spent much of his life studying vascular calcification, you know that perhaps 80%-85% of diabetic patients coming onto dialysis have vascular calcification and maybe 70%-75% of nondiabetic patients have vascular calcification. It's my feeling, and perhaps you agree with me, that the more carefully we look, the higher those rates are, especially if we use more and more sophisticated studies. And we know based on work by Giachelli and others that the high phosphorus that's absorbed, which we spoke about before, can't be excreted and causes the vascular smooth muscle cells to actually transform into bone cells, into osteoblasts. Those osteoblasts lay down collagen, that collagen becomes mineralized. That nice compliant artery that would stretch every time there was systole no longer stretches. The pulse wave velocity goes up. Organs are damaged and we know that patients with calcifications don't live as long as patients without calcifications.[3]
Dr. Raggi: So, it is a very prevalent problem in your dialysis patients, especially, although from my reading, there's an increase in prevalence of calcifications even in earlier ages of CKDcompared with the general population.[4]
Dr. Bushinsky: Absolutely. As I said, the earlier we look, the more sophisticated the technology that you use to look, then the more frequently we find it. It's there.
Dr. Raggi: So that's the importance, therefore, of managing mineral metabolism for the vascular health of your patients. Now, you have a recent publication that I would like to discuss with you, if you don't mind.[5] It's very intriguing but also quite complex, so I hope you can lead me and the audience to understand the mathematics behind it. The topic of your publication is how do we handle (just said in very poor words) calcium? Where do we put it when we absorb it? How do we dispose of it?
Contributions of Intestine, Bone, Kidney, and Dialysis to Extracellular Fluid Calcium Content
Slide 10.
Dr. Bushinsky: I don't think it's complex. It's just simple, simple balance. The body has a tremendous amount of calcium, which is mostly sitting in our bone. But we know if that bone calcium or absorbed calcium moves into the extracellular fluid space, that calcium has to go some place. It can't just stay in the circulation or serum calcium levels will rise and rise, and our patients won't do very well. So that calcium gets deposited in places such as soft tissues and bone. We spoke about how secondary hyperparathyroidism leads bone to release some of its calcium into this systemic circulation, but then it has to go some place. But there are other disorders in CKD that also lead to this calcification. One of them is acidosis.
Dr. Raggi: Right.
Slide 11.

Dr. Bushinsky: Acidemia. We know that metabolic acidosis causes the bone to release calcium, which causes bone formation to go down, bone reabsorption to go up, and the bone breaks down. But that calcium again goes into the extracellular fluid space and since there are no functioning kidneys, where does it go? So we have a second problem, secondary hyperparathyroidism; and thirdly, we as nephrologists, sometimes create a third problem in that we give our patients large amounts of activated vitamin D compounds. And, you know, vitamin D is terrific. We all need vitamin D, but anything in perhaps excess is not so good, because it's going to cause more calcium to be absorbed through the intestine. Remember Jack Coburn showed it goes up. Others have shown intestinal absorption goes up with vitamin D.[6] And high doses of vitamin D can also cause bone to break down, at least in in vitro studies.[7] So, we have 3 things that are adding calcium to the extracellular fluid, the secondary hyperthyroidism, the acidosis, and large amounts of vitamin D. And it can't be lost, because the kidneys can't excrete that calcium.
Dr. Raggi: Interesting. So, all vitamin D preparations are the same as far as the gastrointestinal absorption of calcium or the effect on bone?
Slide 12.
Dr. Bushinsky: Clearly in some rat studies, different vitamin D preparations cause a little less absorption, and some cause a little more absorption. But in high doses, to my knowledge and perhaps to your knowledge, no one's ever shown differences in intestinal absorption in humans. In rats, it's very different. In humans, we don't know.
Dr. Raggi: Of course. Let me ask you to elaborate a little on a couple of cases. In your article, you make the assumption that this is the amount of calcium I'm administering in this patient. I'm either using or not using vitamin D.
Dr. Bushinsky: Right.
Dr. Raggi: Do you want to lead us through 1 or 2 of those cases so that the audience may understand better what you're talking about?
Slide 13.
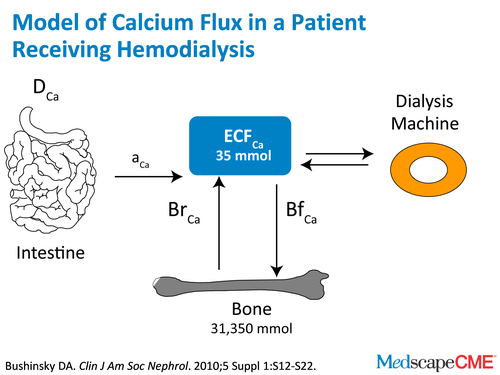
Dr. Bushinsky: In dialysis, we have a dialyzer. And a dialyzer is terrific, because it not only removes waste products but it can add or remove calcium. Now during net ultrafiltration when we remove some of that extra extracellular fluid that our patients have, calcium goes with it. If we use a very low dialysate concentration, you can actually remove calcium from the body. The problem with that, and you as a cardiologist know better than me, is if you try to remove calcium over 3 or 4 hours from the body, you're going to lower serum calcium and you're probably going to induce some cardiac instability.
Dr. Raggi: Sure.
Dr. Bushinsky: Also, we know if we try to lower calcium that way, PTH is going to go up like crazy because it's trying to defend the calcium.
Slide 14.
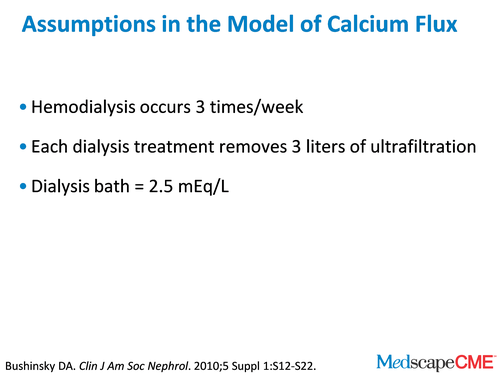
When we mathematically model this disorder of calcium retention in dialysis patients, we assume dialysis is 3 times a week. That's a pretty good assumption. We assume that they're taking off 3 L of ultrafiltration with each dialysis treatment. In some people there's more, and in some people there's less, but it's a pretty good assumption. We're assuming a dialysis bath is 2.5 mEq/L, which is a pretty typical bath in all dialysis units. Now, the key assumption is how much calcium we're going to absorb.
Slide 15.

The best data we have, as I said, is years old, and it's from Jack Coburn. It's 19% of calcium absorbed without activated vitamin D, and a higher level with [activated vitamin D]. In our model we used 25%, but it could be 23% or 27%. It doesn't matter too much, because the data are pretty much the same. We made the assumption that all the vitamin D preparations lead to an equal amount of calcium absorption. They may or may not, but that's all the data we have. We said all the calcium we eat will be absorbed equally, if it comes in from binders, milk or cheese. And again, that's an assumption, but we have to make assumptions or we can't do anything.
Dr. Raggi: Absolutely.
Dr. Bushinsky: And we said the urine calcium was pretty much zero.
Case Example
Slide 16.
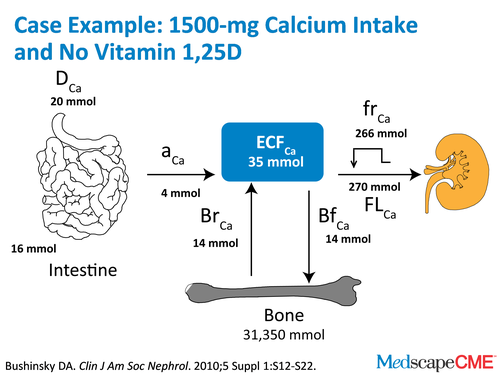
Dr. Bushinsky: So with those assumptions, we can start making predictions and we can predict that if our dialysis patients are ingesting 1.5 g of elemental calcium a day and aren't on any vitamin D, they'll be in pretty neutral balance. This harkens back to the Kidney Disease Outcomes Quality Initiative (KDOQI).
Dr. Raggi: Yes.
Dr. Bushinsky: You remember the recommendations.
Dr. Raggi: Absolutely.
Dr. Bushinsky: They said 1.5 g of binders with 500 mg from diet and you'd be in pretty good calcium balance.[8] That's what we found, I suspect using some of the same assumptions that they used.
Slide 17.
Dr. Bushinsky: We also [evaluated the effects of] added calcium to the diet in the form of food, or binders, or any other source of calcium, and as you know, so many foods now are fortified with calcium.
Dr. Raggi: Absolutely.
Dr. Bushinsky: They're fortified with vitamin D and calcium.
Dr. Raggi: So it must be good, right?
Dr. Bushinsky: Right. In America, if a little is good, more is better.
Dr. Raggi: More is better.
Dr. Bushinsky: Right, right. So more calcium and more vitamin D. Of course, in the Womens' Health Initiative, to get totally off topic, more calcium didn't do much for bones.[9]
Dr. Raggi: Absolutely.
Dr. Bushinsky: Vitamin D caused more kidney stones.
Dr. Raggi: And it wasn't the only publication along those lines.
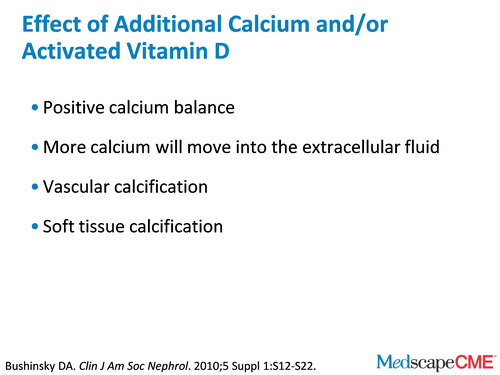
Dr. Bushinsky: Yes, like in the recent British Medical Journal review,[10] but that's another topic for another time. So if our patients are taking 1.5 g of elemental calcium and no vitamin D, they're in pretty good calcium balance. But if they add more calcium to their diet or if we as physicians give them activated vitamin D, which we often do, they will move into a positive calcium balance. More calcium will move into the extracellular fluid and that calcium has to go some place. We talked before about how many of our patients have vascular calcification.
Dr. Raggi: Right.
Dr. Bushinsky: We also know many of our patients have soft tissue calcification. The source of that calcium has to be either the diet or the bone. It can come from no place else.
Slide 18.
Therefore, I think we have to be careful in our dialysis patients. We have to be careful that we don't overload them with calcium. We have to be careful that we don't use copious amounts of phosphate-based binders, because that calcium is absorbed and has to go some place. We have to be careful that we don't use too much vitamin D.
Dr. Raggi: Yes.
Dr. Bushinsky: We should use vitamin D perhaps to replace a missing hormone. I'm not saying we don't need vitamin D. Sure, we need some vitamin D, but more and more vitamin D is perhaps not better and better.
Dr. Raggi: And I want to make sure for the benefit of the audience and even for my benefit, that we don't badger that too much calcium and vitamin D is harmful. Let me ask you the obvious question, aren't we giving vitamin D and calcium for the bone? Are we doing it for the bone if we're not doing it for the vessels? Is there any evidence that we're doing it for the bone?
Dr. Bushinsky: Well, as you know, bone biopsies are very hard to do. There is no real evidence to say that we're saving bone or making bone stronger and better. Do patients need some vitamin D? The retrospective analysis of many databases, but not all databases, suggest that it's beneficial but we still don't have the critical controlled studies to show that it's better.[11] That said, I give my dialysis patients small amounts of vitamin D. I give my dialysis patients small amounts of calcium-based phosphate binders. What we're talking about today is excess. We're not talking about a little bit of vitamin D. Reasonable amounts. We're not talking about not using anything. We're talking about not using excess amounts, because there are no data to demonstrate that they're helpful. At least if we model the system, it may be harmful, because that absorbed calcium has to go some place. It can't go out the urine, because our patients have no calcium in their urine. They have essentially no urine. It doesn't go out in dialysis machines either. Where does it go? We know if I do a bone scan on a dialysis patient, their muscles light up. Their lungs light up. They're being calcified, because their vessels clearly light up. So, use some vitamin D. Use some calcium-based phosphate binders, but use them in moderation.
Dr. Raggi: In closing, I would like for you to elaborate on some final graph that you published in your paper. It was quite helpful to summarize what you had discussed in your paper.
Dr. Bushinsky: Yes.
Dr. Raggi: Can you go over it with us for a second?
Slide 19.
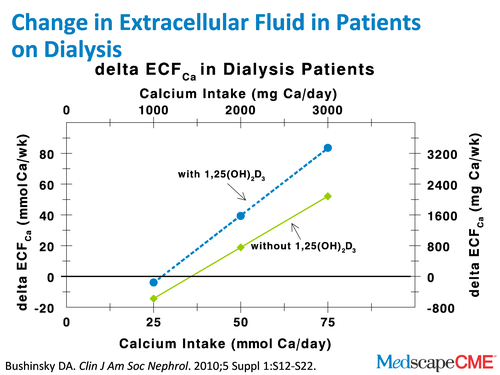
Dr. Bushinsky: Basically, we simply concluded based on those assumptions that we talked about before, that people taking about 1.5 g of elemental calcium without vitamin D don't really move calcium from their bone or their intestine to their extracellular fluid. They're pretty much in neutral calcium balance. But as we add vitamin D and calcium, they move calcium from their intestine and their bone into their extracellular fluid. As I've said, the concentration of calcium can't keep going up. That calcium has to be deposited. At the American Society of Nephrology meeting [November 16-21, Denver, Colorado], where we're taping this from, there's a very nice abstract by David Spiegel at the University of Colorado, and he shows calcium imbalance studies, basically what we've modeled.[12] It's remarkable. It's like our patients are his patients. Read the article. We look forward to seeing that in full print form.
Slide 20.
Dr. Raggi: David, thank you for that lucid discussion. Do you have any closing remarks before we conclude?
Dr. Bushinsky: Paolo, you're very welcome. What we really need in nephrology, not only in this topic but in many others, are randomized controlled trials evaluating plus/minus vitamin D, plus/minus calcium-based binders, and plus/minus noncalcium-based binders with the endpoint being mortality. We, as a country, spend a fortune on binders and vitamin D preparations. Yet, there's a paucity of evidence that we're really helping our patients. I think our studies are based on assumptions and these assumptions must be tested. Otherwise, 5 years from now, you and I will be sitting here saying we're still making these assumptions. So, let's demand that our providers use the studies that will help us practice evidence-based medicine, so we will be able to say to a patient with assurance, "I'm giving this to you, because it saves lives, improves your bone, or decreases vascular calcification." I'd feel much better as a physician if I were able to say that with assurance to patients and not say, "Well, based on mathematical models and assumptions, this is what we should do." So it's a call for randomized placebo-controlled studies.
Slide 21.

Dr. Raggi: Thank you. We've come to the end of our discussion. I hope you found this activity useful to your practice. Thank you for participating in this CME/CE activity. To proceed to the online CME test, click on the Earn CME Credit link on this page. Thank you again for joining us today and thank you, David.
Dr. Bushinsky: Thank you, Paolo. It was a pleasure.
Supported by an independent educational grant from Genzyme.
References
- Rix M, Andreassen H, Eskildsen P, et al. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084-1093. Abstract
- Walser M. The separate effects of hyperparathyroidism, hypercalcemia of malignancy, renal failure, and acidosis on the state of calcium, phosphate, and other ions in plasma. Clin Invest. 1962;41:1454-1471.
- Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453-1464. Abstract
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478-1483. Abstract
- Bushinsky DA. Contribution of intestine, bone, kidney, and dialysis to extracellular fluid calcium content. Clin J Am Soc Nephrol. 2010;5 Suppl 1:S12-22. Abstract
- Gallagher JC, Riggs BL, Eisman J, et al. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979;64:729-736. Abstract
- Armbrecht HJ, Zenser TV, Davis BB. Effect of vitamin D metabolites on intestinal calcium absorption and calcium-binding protein in young and adult rats. Endocrinology. 1980;106:469-475. Abstract
- National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Guideline 5. Use of phosphate binders in CKD. Am J Kidney Dis. 2003;42(4 Suppl 3):S1-S201.
- Jackson RD, LaCroix AZ, Gass M, et al, for the Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683. Abstract
- Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691.
- Palmer SC, McGregor DO, Craig JC, et al. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2009;4:CD008175.
- Spiegel DM & Moore RH. Positive Calcium Balance in CKD. Program and abstracts of the 43rd Annual Meeting & Scientific Exposition of the American Society of Nephrology (ASN) Renal Week; November 16-21, 2010; Denver, Colorado. Poster TH-PO162.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 