Clinical Context
Planning for contraception after delivery is a critical element of family planning, and the US Centers for Disease Control and Prevention (CDC) provides a review of all available contraceptive methods in its current recommendations. Progestin-only hormonal contraceptives are safe to initiate immediately after delivery, and they can be used among women who are breast-feeding. Intrauterine devices (IUDs) can be placed immediately after delivery. Although there is a higher risk for expulsion immediately after delivery, continuation rates of IUDs at 6 months postpartum is similar regardless of when the device is placed. Women should not initiate contraception with a diaphragm or cervical cap until 6 weeks postpartum.
The use of combined hormonal contraception during the postpartum period is a complicated issue, and the current recommendations revise previous guidelines issued by the CDC in 2010.
Study Synopsis and Perspective
The CDC has updated its guidelines for postpartum contraceptive use, according to revised recommendations reported in the July 8 issue of MMWR. Morbidity and Mortality Weekly Report. The new statement, which updates the CDC's U.S. Medical Eligibility Criteria for Contraceptive Use, 2010, advises postpartum women not to use combined hormonal contraceptives during the first 21 days after delivery because of a high risk for venous thromboembolism (VTE).
"The postpartum period is an important time to initiate contraception because women are accessing the health-care system and might have increased motivation to avoid another pregnancy," write Naomi K. Tepper, MD, from the Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC, and colleagues. "Ovulation can occur as early as 25 days postpartum among nonbreastfeeding women, underscoring the importance of initiating contraception in the very early postpartum period."
The revised guidelines affirm the importance of starting contraception during the postpartum period to prevent unintended pregnancy and short birth intervals, which are associated with adverse health outcomes for the mother as well as for the infant. These include greater risks for low birth weight and preterm birth. The 2010 CDC recommendations offered evidence-based guidance for choosing a contraceptive method, considering patient-specific characteristics or medical conditions, such as the postpartum period.
New Guidelines More Stringent
On the basis of subsequent evidence, the World Health Organization (WHO) updated its recommendations regarding the safety of combined hormonal contraceptives among postpartum non–breast-feeding women. Compared with control participants, women in the first 42 days of the postpartum period have a 22-fold to 84-fold increased risk for VTE. The new WHO guidelines were therefore more restrictive concerning use of combined hormonal contraceptives during the first 42 days after delivery, especially in women who had other risk factors for VTE.
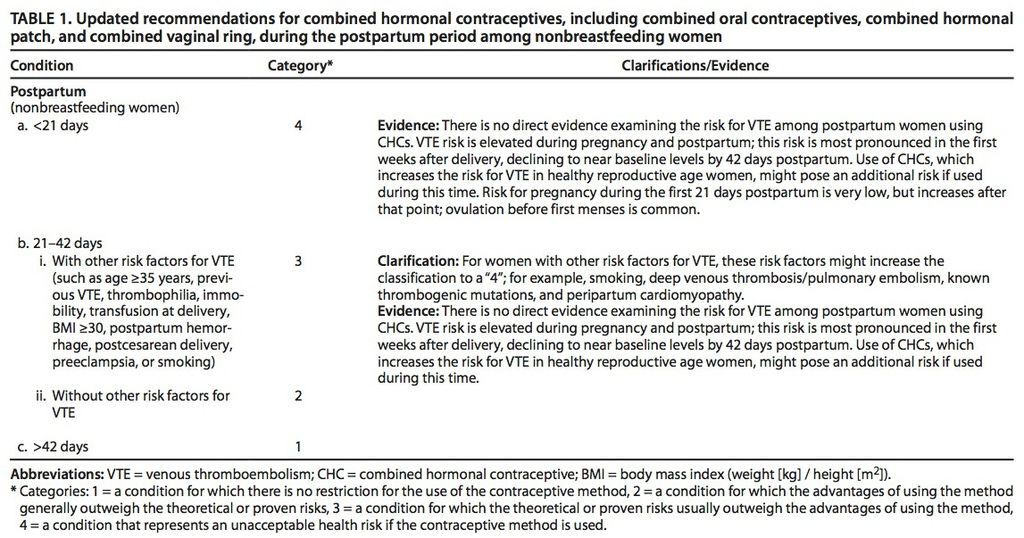
To review the WHO recommendations and underlying evidence base, the CDC therefore convened a group of 13 ad hoc reviewers. On the basis of available evidence concerning the safety of combined hormonal contraceptive use in postpartum women, the CDC has now concluded that the high risk for VTE during the first 21 days after delivery should preclude use of combined hormonal contraceptives in this time frame. Although the risk for pregnancy is very low during the first 21 days postpartum, it increases thereafter, and ovulation before first menses is common.
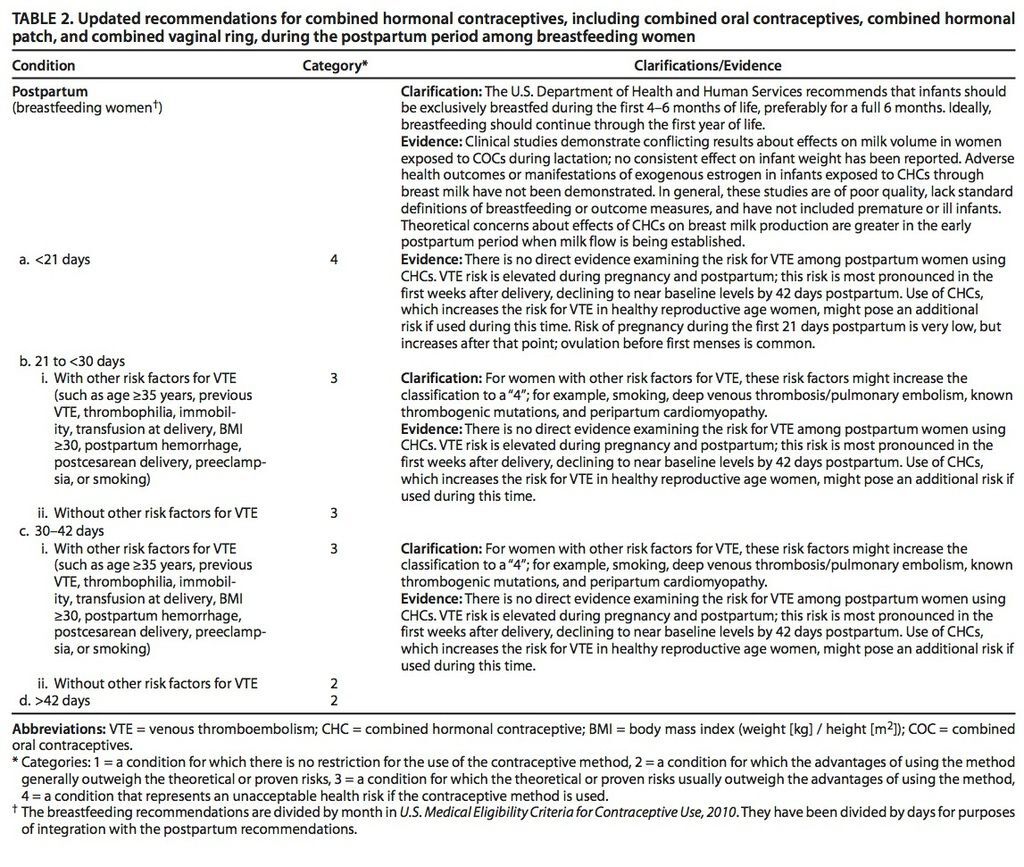
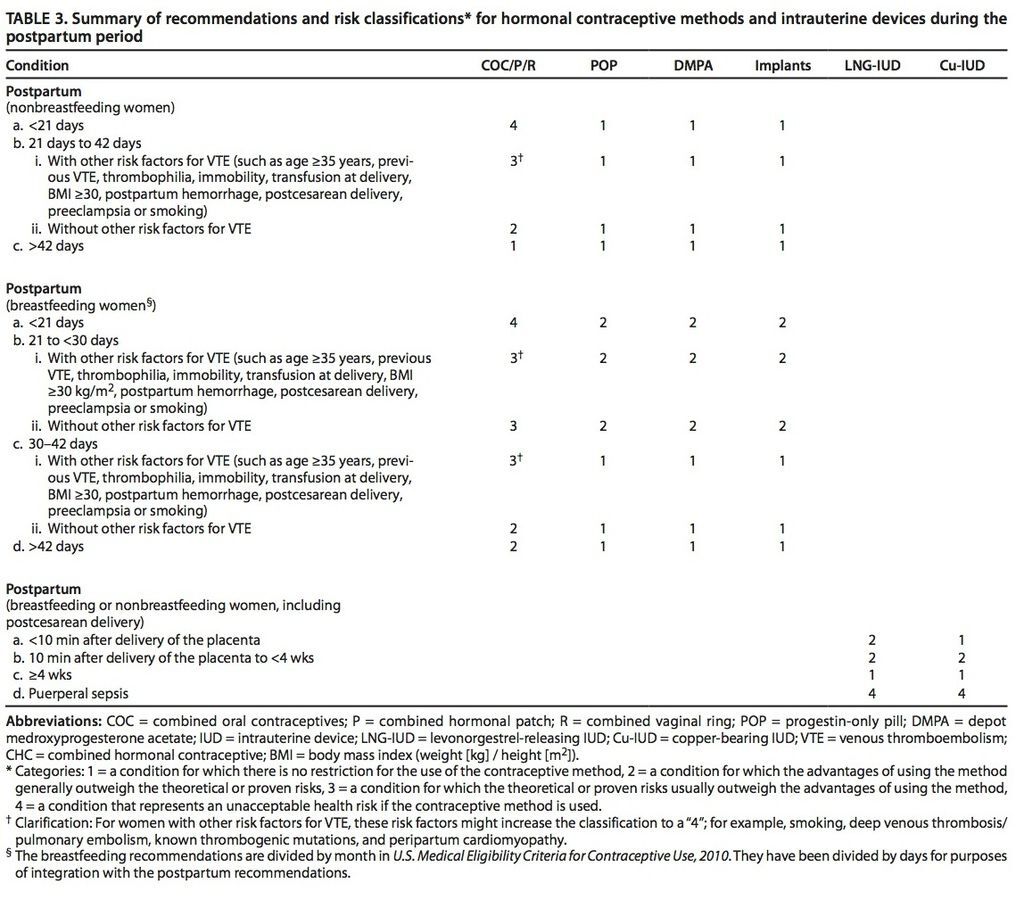
From 21 to 42 days after delivery, women with risk factors for VTE generally should not use combined hormonal contraceptives, but these are thought to be safe during this period for women without risk factors for VTE. Risk factors for VTE include age 35 years or older, previous VTE, thrombophilia, known thrombogenic mutations, immobility, transfusion at delivery, body mass index of at least 30 kg/m2, postpartum hemorrhage, peripartum cardiomyopathy, post cesarean delivery, preeclampsia, or smoking. The CDC has issued no restrictions on the use of combined hormonal contraceptives in women who delivered more than 42 days previously.
"Health-care providers assessing a woman's individual risk also should consider any other characteristics or medical conditions that might impact the classification," the guidelines authors write. "For postpartum women, this might include examining the recommendations for other risk factors for VTE, such as known thrombogenic mutations (category 4) or history of VTE with risk factors for recurrence (category 4), both of which pose an unacceptable health risk for combined hormonal contraceptive use, whether or not women are postpartum."
根據發表在2011年7月8日的MMWR(發病率和死亡率週報)的修訂建議,美國疾病管制和預防中心(CDC)已經更新了他們的產後避孕藥使用指南。此新的聲明更新了CDC 2010年美國避孕藥使用醫療資格標準,建議產後婦女在分娩後前21天內不要使用組合式賀爾蒙避孕藥,因為有靜脈血栓栓塞(venous thromboembolism,VTE)的高風險。CDC目前已做成結論:由於分娩後前21天的高VTE風險,在這段時間內應排除使用組合式荷爾蒙避孕藥。雖然產後前21天懷孕的風險是很低的,但其後會增加,在第一次月經來前排卵是很常見的。
Recommendations on Other Contraceptive Methods
The CDC recommendations are unchanged regarding progestin-only contraceptives (progestin-only pills, depot medroxyprogesterone acetate injections, and implants), IUDs, and contraceptive methods other than combined hormonal contraceptives. These methods can be started immediately after delivery and are safe for postpartum women, including those who are breast-feeding. However, clinicians should consider that combined hormone contraceptives may hinder successful breast-feeding.
Insertion of IUDs, including the levonorgestrel-releasing IUD and copper-bearing IUD, immediately after delivery, is not associated with an increase in complications. Rates of IUD expulsion are somewhat higher when they are inserted within 28 days of delivery, but continuation rates at 6 months are similar regardless of whether IUD insertion takes place immediately postpartum or is delayed.
Condoms can be safely used at any time, but use of the diaphragm and cervical cap should be delayed to 6 weeks postpartum. Another option in women who have completed their childbearing is to consider postpartum sterilization.
The CDC guidelines authors expressed concern regarding access to contraceptive methods. Unlike progestin implants, IUDs, and other methods requiring a follow-up visit to a provider, combined hormonal contraceptives could be started by the woman herself at the appropriate time if she is issued a prescription or is given a sample in advance, either before hospital discharge or at a postpartum visit.
"Postpartum contraception is important for the health of mother and infant, and education for both health-care providers and women should focus on the range of contraception options and the safety of most of these methods during the postpartum period," the guidelines authors conclude.
MMWR Morb Mortal Wkly Rep. 2011;60:878-883.
Related Link The CDC’s Guidelines for Postpartum Contraceptive Use are available online.
Study Highlights
- The CDC convened a group of 13 ad hoc reviewers to evaluate current recommendations for postpartum contraception from the WHO.
- Ovulation can occur as early as 25 days postpartum among non–breast-feeding women, although fertile ovulation will not usually occur until at least 42 days after delivery.
- Short interpregnancy interval is associated with a higher risk for low birth weight and preterm birth.
- Competing with these issues is that the risk for VTE is elevated by 22-fold to 84-fold in women during the first 42 days of the postpartum period vs control participants. This risk is particularly high in the 3 weeks after delivery.
- Women with other risk factors for VTE, such as advanced maternal age, smoking, or cesarean delivery, carry an even higher risk for postpartum VTE.
- Nonetheless, there is no direct evidence examining the risk for VTE among postpartum women using combined hormonal contraceptives.
- The current recommendations strongly discourage the use of combined oral contraceptives during the first 21 days after delivery. The risk for pregnancy is low during this period, but the risk for VTE is significantly elevated.
- Combined hormonal contraceptives may be initiated between 21 and 42 days postpartum among women without other risk factors for VTE. However, among women with a risk factor, including age 35 years or older, previous VTE, immobility, transfusion after delivery, obesity, smoking, or postpartum hemorrhage, the use of combined hormonal contraceptives should be avoided until at least 42 days postpartum.
- After 42 days postpartum, there are no restrictions on the use of combined hormonal contraceptives based on postpartum status.
- Clinicians should also bear in mind that combined hormone contraceptives can interfere with successful breast-feeding.
Clinical Implications
Progestin-only hormonal contraceptives and the IUD may be initiated immediately after delivery, whereas women should not initiate contraception with a diaphragm or cervical cap until 6 weeks postpartum.
The current recommendations strongly discourage the initiation of combined hormonal contraceptives in the first 21 days postpartum among all women. Women without any risk factor for VTE may initiate combined hormonal contraceptives between 21 and 42 days postpartum, but women with a risk factor for VTE should wait until more than 42 days postpartum.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 