
This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies is then presented, followed by a review of formal guidelines, when they exist. The article ends with the authors' clinical recommendations.
A 35-year-old woman who has never been pregnant and who has a 5-year history of hypertension wants to become pregnant. She has stopped using contraception. Her only medication is lisinopril at a dose of 10 mg per day. Her blood pressure is 124/68 mm Hg, and her body-mass index (the weight in kilograms divided by the square of the height in meters) is 27. What would you advise?
THE CLINICAL PROBLEM
Chronic hypertension in pregnancy is defined as a blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic pressure before pregnancy or, for women who first present for care during pregnancy, before 20 weeks of gestation. The prevalence of chronic hypertension in pregnancy in the United States is estimated to be as high as 3%1 and has been increasing over time. This increase in prevalence is primarily attributable to the increased prevalence of obesity, a major risk factor for hypertension, as well as the delay in childbearing to ages when chronic hypertension is more common. Therefore, an increasing number of women enter pregnancy with hypertension and need both counseling regarding the risks of chronic hypertension in pregnancy and adjustment of antihypertensive treatment before and during pregnancy.
Most women with chronic hypertension have good pregnancy outcomes, but these women are at increased risk for pregnancy complications, as compared with the general population. The risk of an adverse outcome increases with the severity of hypertension and end-organ damage.2 Furthermore, some antihypertensive agents carry risks in pregnancy and should be discontinued before conception.3 Since nearly 50% of pregnancies in the United States are unplanned,4 it is important to counsel women of reproductive age who have hypertension regarding such risks as part of routine care.
Women with chronic hypertension have an increased frequency of preeclampsia (17 to 25%,1,5,6vs. 3 to 5% in the general population), as well as placental abruption, fetal growth restriction, preterm birth, and cesarean section. The risk of superimposed preeclampsia increases with an increasing duration of hypertension.2 Preeclampsia is a leading cause of preterm birth and cesarean delivery in this population.6,7 In a study involving 861 women with chronic hypertension, preeclampsia developed in 22%, and the condition occurred in nearly half these women at less than 34 weeks of gestation, earlier than is typical in women without antecedent hypertension. Women with chronic hypertension with superimposed preeclampsia are at increased risk for giving birth to an infant who is small for gestational age6 and for placental abruption, as compared with women with chronic hypertension without superimposed preeclampsia.
Even in the absence of superimposed preeclampsia, women with chronic hypertension have an increased risk of adverse outcomes.5 Studies that were performed in Canada, the United States, and New Zealand have indicated that fetal growth restriction (estimated or actual fetal weight, <10th percentile for population norms) complicates 10 to 20% of such pregnancies.1,5,6 In an analysis of the Danish National Birth Cohort, after adjustment for age, body-mass index, smoking status, parity, and diabetes, chronic hypertension was associated with approximately five times the risk of preterm birth and a 50% increase in the risk of giving birth to an infant who is small for gestational age.8 Women with chronic hypertension have more than twice the frequency of placental abruption as normotensive women (1.56% vs. 0.58%),9 a risk that is further increased in women with preeclampsia.2,9 Chronic hypertension has also been associated with an increased risk of stillbirth.10
Most women with chronic hypertension have a decrease in blood pressure during pregnancy, similar to that observed in normotensive women; blood pressure falls toward the end of the first trimester and rises toward prepregnancy values during the third trimester. 5,11,12 As a result, antihypertensive medications can often be tapered during pregnancy. However, in addition to the subset of women with chronic hypertension in whom preeclampsia develops, another 7 to 20% of women have worsening of hypertension during pregnancy without the development of preeclampsia.13
Prepregnancy Evaluation
The care of women with chronic hypertension should begin before pregnancy in order to optimize treatment regimens before conception and facilitate counseling concerning potential pregnancy complications. Prepregnancy evaluation of chronic hypertension should generally follow the guidelines of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure 7 (JNC 7) for assessment of target-organ damage, recommendations that do not include specific modifications for evaluation during pregnancy.14 Such recommendations include the use of electrocardiography and assessment of blood glucose, hematocrit, serum potassium, creatinine, calcium, and lipoprotein profile, as well as urinalysis. Given the increased risk of preeclampsia in women with chronic hypertension, evaluation before pregnancy should also include a 24-hour quantification of urine protein to facilitate the identification of subsequent superimposed preeclampsia. The presence of end-organ manifestations of hypertension may worsen the prognosis during pregnancy and should be taken into account in counseling. For example, the presence of proteinuria at baseline increases the risks of superimposed preeclampsia and growth restriction.2
In most women with chronic hypertension, the cause of the disorder is unknown. The rate of identifiable causes of hypertension in women of childbearing age is not well studied. The evaluation of identifiable causes of hypertension is generally limited to women with hypertension that is resistant to therapy or that requires multiple medications or to those who have symptoms or signs that suggest a secondary cause; evaluations in such cases should follow the JNC 7 guidelines.14However, because testing in these cases may require the diagnostic use of radiation and because the treatment of detected abnormalities often includes surgery, practitioners should pursue such evaluations before conception whenever possible.
Monitoring for Preeclampsia
Identifying superimposed preeclampsia in women with chronic hypertension can be challenging, given that blood pressures are high to start and some women may have baseline proteinuria. Superimposed preeclampsia should always be considered when the blood pressure increases in pregnancy or when there is a new onset of or an increase in baseline proteinuria. An elevated uric acid level may help to distinguish the two conditions, although there is substantial overlap in levels. The presence of thrombocytopenia or elevated values on liver-function testing may also support a diagnosis of preeclampsia. Recently, serum and urinary angiogenic markers have been studied as possible aids in the diagnosis of superimposed preeclampsia,15 but data are currently insufficient to support their use in this population.
Antihypertensive Medications
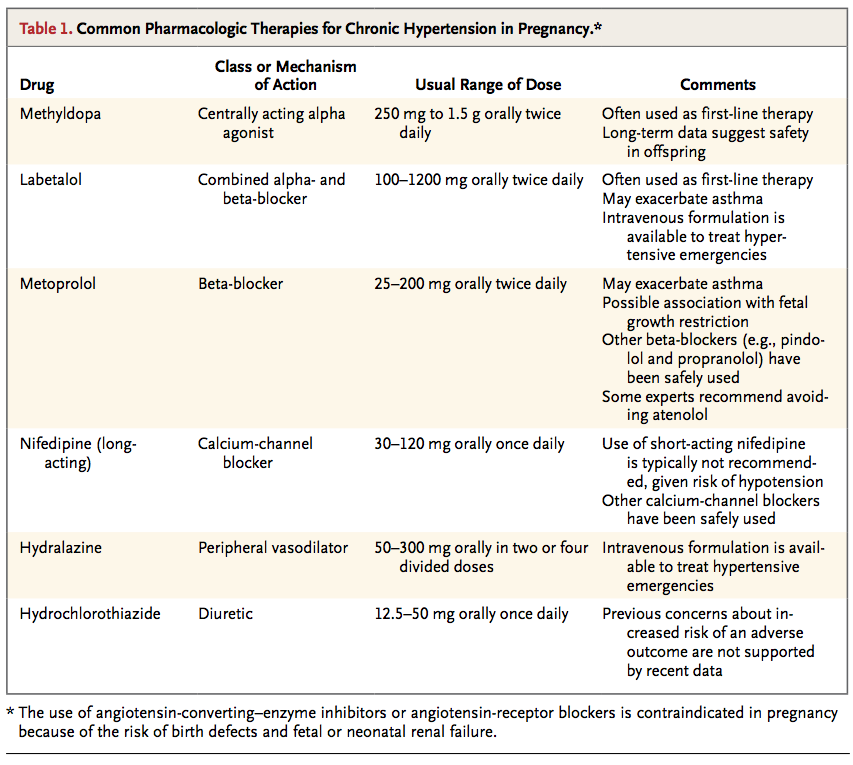
A primary reason for treating hypertension in pregnancy is to reduce maternal morbidity associated with severe hypertension (Table 1)
A meta-analysis including 28 randomized trials comparing antihypertensive treatment either with placebo or with no treatment showed that antihypertensive treatment significantly reduced the risk of severe hypertension. However, treatment did not reduce the risks of superimposed preeclampsia, placental abruption, or growth restriction, nor did it improve neonatal outcomes.13
The antihypertensive agent with the largest quantity of data regarding fetal safety is methyldopa, which has been used during pregnancy since the 1960s. In one study,16 no adverse developmental outcomes were reported during 7.5 years of follow-up among 195 children whose mothers had received methyldopa. Thus, methyldopa is considered to be a first-line therapy in pregnancy by many guideline groups.17-19 However, methyldopa frequently causes somnolence, which may limit its tolerability and necessitate the use of other agents.
In a meta-analysis of randomized trials comparing different antihypertensive agents in pregnancy, the use of beta-blockers resulted in fewer episodes of severe hypertension than the use of methyldopa.13 Labetalol, a combined alpha- and beta-receptor blocker, is often recommended as another first-line18 or second-line17 therapy for hypertension in pregnancy. Although some data have suggested an association between atenolol and fetal growth restriction, 20 this finding has not been reported with the use of other beta-blockers or labetalol, and whether the observed association was attributable to the use of atenolol or to the underlying hypertension is uncertain. Nonetheless, some experts consider it prudent to avoid the use of atenolol during pregnancy.18
Long-acting calcium-channel blockers also appear to be safe in pregnancy, although experience is more limited than with labetalol.21 Diuretics were long considered contraindicated in pregnancy because of concern about volume depletion. However, a review of nine randomized trials showed no significant difference in pregnancy outcomes among women with hypertension who took diuretics and those who took no antihypertensive medication.22 Accordingly, some guidelines support the continuation of diuretic therapy during pregnancy in women with chronic hypertension who were previously treated with these agents.17,18
Angiotensin-converting–enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) are contraindicated in pregnancy. Their use in the second half of pregnancy has been associated with oligohydramnios (probably resulting from impaired fetal renal function) and neonatal anuria, growth abnormalities, skull hypoplasia, and fetal death.23-26 ACE inhibitors have also been associated with potential teratogenic effects. In a retrospective cohort study that included women who had been exposed to ACE inhibitors in the first trimester, the risk ratio associated with exposure to ACE inhibitors, as compared with exposure to other antihypertensive agents, was 4.0 (95% confidence interval [CI], 1.9 to 7.3) for cardiovascular defects and 5.5 (95% CI, 1.7 to 17.6) for central-nervous-system defects.3 Although the observational nature of the study makes it impossible to rule out confounding by other factors associated with the use of ACE inhibitors, it is recommended that women taking ACE inhibitors and, by extrapolation, other blockers of the renin–angiotensin system (e.g., ARBs and renin inhibitors) be switched to another antihypertensive class of drugs before conception whenever possible.
Lifestyle modifications, including weight reduction and increased physical activity, have been shown to improve blood-pressure control in persons who are not pregnant. In addition, an increased body-mass index is a well-established risk factor for preeclampsia.27 The American College of Obstetrics and Gynecology recommends weight reduction before pregnancy in obese women.28However, data are lacking to inform whether such measures improve pregnancy outcomes specifically in women with hypertension.
Blood-Pressure Goals in Pregnancy
In the absence of conclusive data from randomized trials to guide thresholds for initiating the use of antihypertensive medications or blood-pressure targets in pregnancy, various professional guidelines provide disparate recommendations regarding indications for starting therapy (ranging from a blood pressure of >159/89 mm Hg14,17,29 to one of >169/109 mm Hg18,19) and for blood-pressure targets for women who are receiving therapy (ranging from <140/90 mm Hg29 to <160/110 mm Hg14). Some experts recommend stopping antihypertensive agents during pregnancy, as long as blood pressures fall below such thresholds. For women whose antihypertensive therapy is continued, aggressive lowering of blood pressure should be avoided. A meta-analysis of randomized trials of antihypertensive treatment of mild-to-moderate hypertension in pregnancy (both chronic and pregnancy-associated) suggested that a greater magnitude of blood pressure lowering was associated with an increased risk of fetal growth restriction.30 Accordingly, prepregnancy doses of antihypertensive agents may need to be reduced, particularly in the second trimester, when blood pressures typically fall with respect to levels before pregnancy or during the first trimester.
Prevention of Preeclampsia
Since superimposed preeclampsia is the major adverse pregnancy outcome associated with chronic hypertension, many women ask whether any therapies can decrease this risk. Large, randomized, placebo-controlled trials have shown no significant reduction in the risk of preeclampsia associated with the use of low-dose aspirin,31 calcium supplementation,32 or antioxidant supplementation with vitamins C and E,33 although meta-analyses of smaller studies suggested benefit.34,35
Fetal Surveillance
Efforts to monitor women and their fetuses for complications may include more frequent prenatal visits for women with chronic hypertension than for women without this condition. Such visits are intended to monitor women closely for complications of chronic hypertension by measuring blood pressure and urine protein. Because such pregnancies have an increased likelihood of fetal growth restriction, the evaluation of fetal growth is recommended. Many obstetricians supplement regular evaluation of fundal height with ultrasonographic estimation of fetal weight, beginning in the early third trimester and continuing at intervals of 2 to 4 weeks, depending on maternal blood pressure, medications, complications, and findings on previous imaging. Although data from low-risk populations suggest that ultrasonography and evaluation of fundal height have shown similar results for the detection of growth restriction,36 ultrasonography also assesses amniotic-fluid volume and fetal movements and tone (biophysical profile), evaluations that may be useful with respect to the risks associated with chronic hypertension in pregnancy.
Given the increased risk of stillbirth in mothers with hypertension,10 surveillance of fetal well-being is also recommended by some experts, although others recommend restricting such testing to pregnancies with complications, such as growth restriction or preeclampsia.17,18 Testing may also include the evaluation of the pattern and variability of the fetal heart rate (nonstress testing). Maternal complications (e.g., preeclampsia or worsening hypertension), nonreassuring fetal-testing results, or concern about fetal growth restriction are often indications for early delivery. Clinicians must weigh the risks of fetal morbidity associated with delivery before term against the risks of maternal and fetal complications from continued expectant management. In women with chronic hypertension without additional complications, delivery is often planned near the estimated due date, although the need for such intervention is uncertain if testing results are reassuring and fetal growth is normal.
Breast-Feeding
Breast-feeding should be encouraged in women with chronic hypertension, including those requiring medication. Although most antihypertensive agents can be detected in breast milk, levels are generally lower than those in maternal plasma.37 These relatively low levels and observational data from series of women who were receiving medications while breast-feeding have led the American Academy of Pediatrics to label most antihypertensive agents, including ACE inhibitors, as “usually compatible” with breast-feeding. 38 Since case reports have described lethargy and bradycardia in newborns who are breast-fed by mothers taking atenolol, the American Academy of Pediatrics recommends that atenolol be used “with caution.” No such cautions are noted for other beta-blockers, such as metoprolol. Because data are lacking with respect to the use of ARBs and breast-feeding, it is recommended that other agents be considered for treating hypertension in lactating women. Recommendations from the Society of Obstetricians and Gynaecologists of Canada note that the use of long-acting nifedipine, labetalol, methyldopa, captopril, and enalapril is acceptable during breast-feeding. 21
AREAS OF UNCERTAINTY
Data from randomized trials to inform the treatment of women with chronic hypertension in pregnancy are limited, including whether women with mild-to-moderate hypertension should receive antihypertensive treatment, which target blood pressure should be used for treatment, and which antihypertensive agents are superior for use in pregnancy. The Control of Hypertension in Pregnancy Study (CHIPS; ClinicalTrials.gov number, NCT01192412) is an ongoing randomized trial involving women with chronic hypertension or pregnancy-associated hypertension that is comparing “less tight” control (target diastolic blood pressure, 100 mm Hg) with “tight” control (target diastolic blood pressure, 85 mm Hg) with respect to maternal, fetal, and neonatal outcomes39; study completion is anticipated in 2013. Additional prospective studies are needed to assess maternal and fetal outcomes associated with the use of different antihypertensive therapies and blood-pressure targets. Long-term follow-up of both mother and offspring is also warranted, particularly given the increasing evidence that the environment in utero influences later health outcomes.40
GUIDELINES
Guidelines for the management of pregnancy in women with chronic hypertension have been published by the American College of Obstetricians and Gynecologists,18 the Society of Obstetricians and Gynaecologists of Canada,41 the Working Group of the National High Blood Pressure Education Program,17 and the Australasian Society for the Study of Hypertension in Pregnancy29 (Table 2)
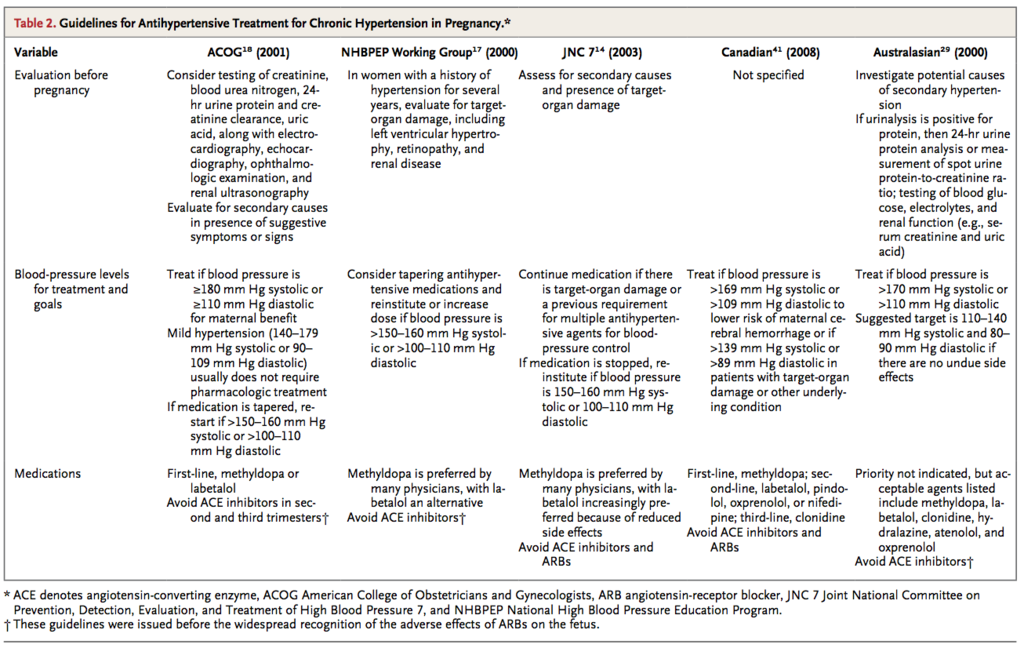
These guidelines all emphasize the importance of preconception planning and management, recommend that ACE inhibitors be avoided in pregnancy, and emphasize the long experience supporting the safety of methyldopa during pregnancy. However, different guidelines suggest disparate thresholds for antihypertensive treatment and differ in recommendations regarding certain medications, including whether they endorse the use of atenolol in pregnancy.
CONCLUSIONS AND RECOMMENDATIONS
The woman with hypertension who is described in the vignette should be counseled to use contraception until she has undergone a prepregnancy evaluation, including assessment of end-organ damage; evaluation for identifiable causes of hypertension, if suggested by her medical history, physical examination, or laboratory testing; and adjustment of antihypertensive therapy. If a reversible cause of hypertension is identified, it should be addressed before pregnancy. Before attempting to conceive, the patient should replace the ACE inhibitor with another antihypertensive agent that is considered to be safe in pregnancy (methyldopa, labetalol, or a long-acting calcium-channel blocker), and she should be counseled regarding weight reduction. Although some guidelines recommend the first-line use of methyldopa on the basis of its long safety record, we would generally use labetalol first, since data also support its safety, and in practice we find it to be more effective and to have fewer side effects than methyldopa.
The patient should be closely followed during her pregnancy and be educated regarding the potential risks of chronic hypertension in pregnancy. Since she has a 5-year history of hypertension, she is at increased risk for superimposed preeclampsia.2 In the absence of definitive recommendations with respect to optimal blood-pressure targets during pregnancy, we aim to adjust medications to maintain blood pressure between 130/80 mm Hg and 150/100 mm Hg. Given the need for careful prepregnancy planning and for coordinated care during and after pregnancy for women with chronic hypertension during their reproductive years, we recommend interdisciplinary care involving clinicians who are trained in obstetrics and gynecology and those who are trained in internal or family medicine.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 