Rheumatoid arthritis is a common autoimmune disease that is associated with progressive disability, systemic complications, early death, and socioeconomic costs.1 The cause of rheumatoid arthritis is unknown, and the prognosis is guarded. However, advances in understanding the pathogenesis of the disease have fostered the development of new therapeutics, with improved outcomes. The current treatment strategy, which reflects this progress, is to initiate aggressive therapy soon after diagnosis and to escalate the therapy, guided by an assessment of disease activity, in pursuit of clinical remission.
However, several unmet needs remain. Current conventional and biologic disease-modifying therapies sometimes fail or produce only partial responses. Reliable predictive biomarkers of prognosis, therapeutic response, and toxicity are lacking. Sustained remission is rarely achieved and requires ongoing pharmacologic therapy. The mortality rate is higher among patients with rheumatoid arthritis than among healthy persons, and cardiovascular and other systemic complications remain a major challenge. Molecular remission and the capacity to reestablish immunologic tolerance remain elusive. Elucidation of the pathogenic mechanisms that initiate and perpetuate rheumatoid arthritis offers the promise of progress in each of these domains. Rheumatoid arthritis is predominantly classified on the basis of the clinical phenotype.2 We believe it is important to make the transition to a new molecular taxonomy that defines discrete disease subgroups with distinct prognostic and therapeutic significance.3
Rheumatoid arthritis is characterized by synovial inflammation and hyperplasia (“swelling”), autoantibody production (rheumatoid factor and anti–citrullinated protein antibody [ACPA]), cartilage and bone destruction (“deformity”), and systemic features, including cardiovascular, pulmonary, psychological, and skeletal disorders. These clinical features pose critical mechanistic questions: What genetic–environmental interactions must occur to facilitate autoimmunity a priori, and why does this beget articular localization? Why does synovial inflammation perpetuate? What drives local destruction leading to joint dysfunction? Why does rheumatoid arthritis cause systemic illness? We herein summarize key pathogenetic advances informing these issues.
GENETIC AND ENVIRONMENTAL FACTORS
Rheumatoid arthritis involves a complex interplay among genotype, environmental triggers, and chance. Twin studies implicate genetic factors in rheumatoid arthritis, with concordance rates of 15 to 30% among monozygotic twins and 5% among dizygotic twins.4 Genomewide analyses make it clear that immune regulatory factors underlie the disease.5 The long-established association with the human leukocyte antigen (HLA)–DRB1 locus has been confirmed in patients who are positive for rheumatoid factor or ACPA; alleles that contain a common amino acid motif (QKRAA) in the HLA-DRB1 region, termed the shared epitope, confer particular susceptibility.6 These findings suggest that some predisposing T-cell repertoire selection, antigen presentation, or alteration in peptide affinity has a role in promoting autoreactive adaptive immune responses. Other possible explanations for the link between rheumatoid arthritis and the shared epitope include molecular mimicry of the shared epitope by microbial proteins, increased T-cell senescence induced by shared epitope–containing HLA molecules, and a potential proinflammatory signaling function that is unrelated to the role of the shared epitope in antigen recognition.
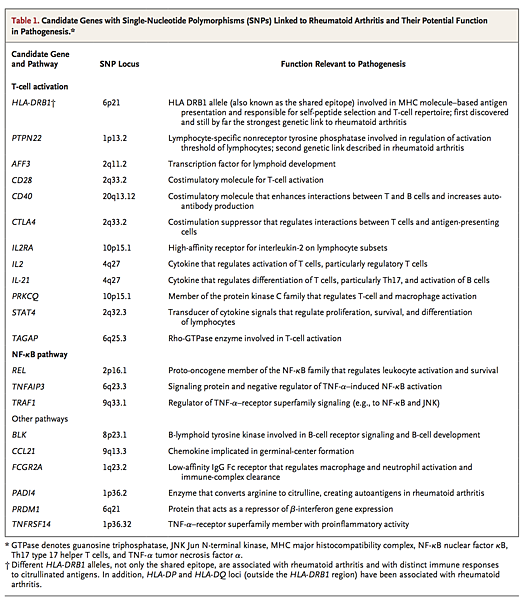
Findings from studies of gene–environment interactions complement these observations. Smoking and other forms of bronchial stress (e.g., exposure to silica) increase the risk of rheumatoid arthritis among persons with susceptibility HLA–DR4 alleles.14 Moreover, smoking and HLA-DRB1 alleles synergistically increase one's risk of having ACPA.15 Unifying these observations is the finding that environmental stressors of pulmonary and other barrier tissues may promote post-translational modifications, through peptidyl arginine deiminase, type IV (PADI4), that result in quantitative or qualitative alteration in citrullination of mucosal proteins.
Infectious agents (e.g., Epstein–Barr virus, cytomegalovirus, proteus species, and Escherichia coli) and their products (e.g., heat-shock proteins) have long been linked with rheumatoid arthritis, and although unifying mechanisms remain elusive, some form of molecular mimicry is postulated.20,21 The formation of immune complexes during infection may trigger the induction of rheumatoid factor, a high-affinity autoantibody against the Fc portion of immunoglobulin, which has long served as a diagnostic marker of rheumatoid arthritis and is implicated in its pathogenesis. Furthermore, rheumatoid arthritis appears to be associated with periodontal disease: Porphyromonas gingivalis expresses PADI4, which is capable of promoting citrullination of mammalian proteins.22 Finally, the gastrointestinal microbiome is now recognized to influence the development of autoimmunity in articular models, and specific (and potentially tractable) clinical bacterial signatures that are associated with autoantibody-positive rheumatoid arthritis are emerging.23
The greater risk of rheumatoid arthritis among women than among men has long been recognized. The onset of rheumatoid arthritis is also associated with adverse life events. Molecular explanations for such phenomena are emerging from animal models of inflammation, which show a link between the hypothalamic–pituitary–adrenal axis and cytokine production.24 The central nervous system is normally involved in immune regulation and homeostasis, and neuroimmunologic interactions regulate disease development in rodent models of arthritis. Such effects may operate locally (several neurotransmitters are expressed in synovitis in rheumatoid arthritis) or centrally (cytokines are rapidly up-regulated in the hypothalamus during peripheral inflammation). Translation of these observations to effective treatment of rheumatoid arthritis is challenging.
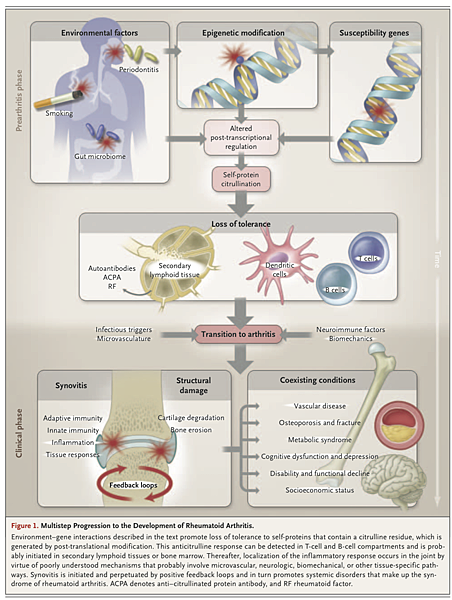
Critical issues remain unresolved. Autoantibodies, such as rheumatoid factor and ACPA, are often (but not always) detected in patients before the development of arthritis (prearticular phase of rheumatoid arthritis); in some series, autoantibody levels have increased and there has been evidence of epitope spreading as the onset of disease approaches.25 Why the systemic loss of tolerance is linked to a localized onset of inflammation in the joint is still unclear (transitional phase of rheumatoid arthritis). It is possible that biologic features of the targeted autoantigen (e.g., regulation of cellular metabolism in the case of α-enolase and glucose-6-phosphatase) may contribute. Other possible factors include local microvascular, neurologic, biomechanical, and microtrauma-related mechanisms
SYNOVIAL IMMUNOLOGIC PROCESSES AND INFLAMMATION
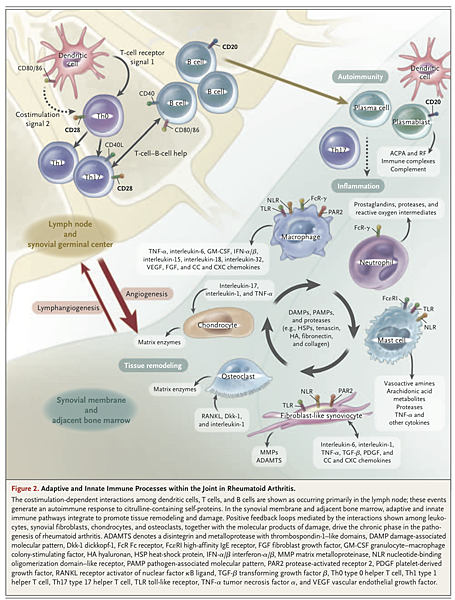
Synovitis occurs when leukocytes infiltrate the synovial compartment. Leukocyte accumulation primarily reflects migration rather than local proliferation. Cell migration is enabled by endothelial activation in synovial microvessels, which increases the expression of adhesion molecules (including integrins, selectins, and members of the immunoglobulin superfamily) and chemokines. Accordingly, neoangiogenesis, which is induced by local hypoxic conditions and cytokines, and insufficient lymphangiogenesis, which limits cellular egress, are characteristic features of early and established synovitis.26,27 These microenvironmental changes, combined with profound synovial architectural reorganization and local fibroblast activation, permit the buildup of synovial inflammatory tissue in rheumatoid arthritis
Adaptive Immune Pathways
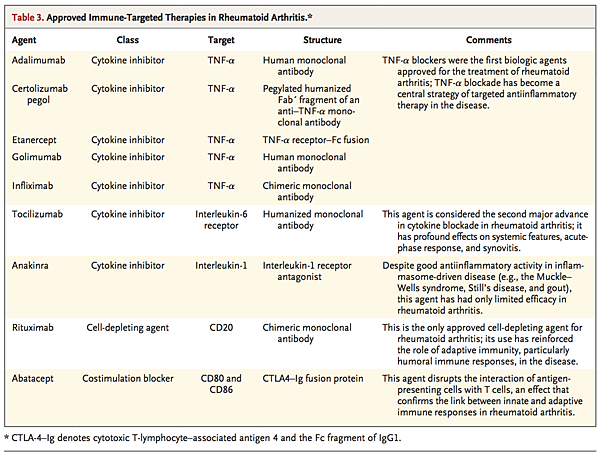
The genetics of rheumatoid arthritis and the presence of autoantibodies clearly place adaptive immunity at the center of early pathogenesis. However, even though T cells are abundant in the synovial milieu, the functional role of T cells remains insufficiently understood. Direct targeting of T cells by cyclosporine or T-cell–depleting therapeutics has shown limited or no efficacy.28 This finding may reflect “broad spectrum” deletion of regulatory as well as effector T cells and suggests the need to target T-cell subsets. The synovium in rheumatoid arthritis contains abundant myeloid cells and plasmacytoid dendritic cells that express cytokines (interleukin-12, 15, 18, and 23), HLA class II molecules, and costimulatory molecules that are necessary for T-cell activation and antigen presentation.29,30 Moreover, the use of abatacept (a fusion protein containing cytotoxic T-lymphocyte–associated antigen 4 and the FC fragment of IgG1) to disrupt antigen presentation by blocking T-cell costimulation (through the interaction of CD28 with CD80 or CD86) is efficacious in rheumatoid arthritis. Autoreactive T cells against citrullinated self-proteins have been identified. Synovial T-cell oligoclonality, germinal-center reactions, and B-cell hypermutation suggest ongoing local antigen-specific, T-cell–mediated B-cell help.
Humoral adaptive immunity is integral to rheumatoid arthritis. Synovial B cells are mainly localized in T-cell–B-cell aggregates — indeed, some tissues have ectopic lymphoid follicles39 — that are supported by the expression of factors that include a proliferation-inducing ligand (APRIL), B-lymphocyte stimulator (BLyS), and CC and CXC chemokines (e.g., CXC chemokine ligand 14 and CC chemokine ligand 21).40 Plasmablasts and plasma cells are more widely distributed in the synovium and also in juxta-articular bone marrow. A pathogenic role for CD20+ B cells is confirmed by the efficacy of rituximab in rheumatoid arthritis.41 Because plasma cells are not targeted by anti-CD20 antibodies, and autoantibody levels are variably altered after treatment, these clinical observations suggest that the role of B cells and their progeny in the pathogenesis of rheumatoid arthritis goes beyond autoantibody production to include autoantigen presentation and cytokine production (e.g., interleukin-6, TNF-α, and lymphotoxin-β).
Activation of the Innate Immune System
A variety of innate effector cells, including macrophages, mast cells, and natural killer cells, are found in the synovial membrane, whereas neutrophils reside mainly in synovial fluid. Macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and granulocyte–macrophage colony-stimulating factor (GM-CSF) enhance maturation of these cells, their efflux from the bone marrow, and trafficking to the synovium.42 In particular, macrophages are central effectors of synovitis; clinically effective biologic agents consistently reduce macrophage infiltration in the synovium.43 Macrophages act through release of cytokines (e.g., TNF-α and interleukin-1, 6, 12, 15, 18, and 23), reactive oxygen intermediates, nitrogen intermediates, production of prostanoids and matrix-degrading enzymes, phagocytosis, and antigen presentation.
This pattern of expression of proinflammatory cytokines and inducible nitric oxide synthase suggests a predominant M1 macrophage phenotype. Macrophages are activated by toll-like receptors (TLRs) (e.g., TLR 2/6, 3, 4, and 8) and nucleotide-binding oligomerization domain (NOD)–like receptors (NLRs) that recognize a range of pathogen-associated molecular patterns and damage-associated molecular patterns that potentially include bacterial, viral, and putative endogenous ligands.44 Macrophage activation is also driven by cytokines, cognate interactions with T cells, immune complexes, lipoprotein particles and liver X–receptor agonists (e.g., oxysterols, oxidized low-density lipoprotein [LDL], and serum amyloid A–rich high-density lipoprotein [HDL]), and the protease-rich microenvironment through protease-activated receptor 2.45 Moreover, microRNA species (e.g., microRNA-155) have been implicated in the regulation of synovial cytokine expression.46,47
Neutrophils contribute to synovitis by synthesizing prostaglandins, proteases, and reactive oxygen intermediates.48 Mast cells that produce high levels of vasoactive amines, cytokines, chemokines, and proteases, through ligation of TLR, suppression of tumorigenicity 2 (ST2), Fc receptor γ, and Fc receptor ε, also play a role.49,50 A fraction of ACPA belongs to the IgE class, which may elicit mast-cell activation through Fc receptor ε.51 These findings, which provide evidence that activation of the innate immune pathway contributes to synovitis, could lead to the development of treatments that modulate TLR-dependent, NLR-dependent, and inflammasome-dependent pathways.
Cytokines and Intracellular Signaling Pathways
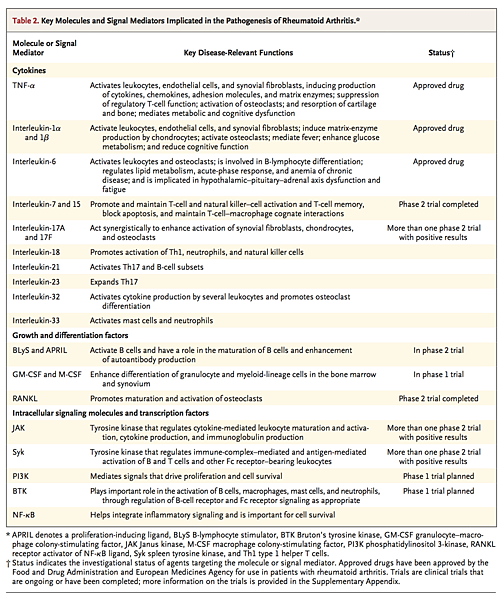
Cytokine production that arises from numerous synovial cell populations is central to the pathogenesis of rheumatoid arthritis. Cytokine patterns may shift over time; early rheumatoid arthritis has an apparently distinct cytokine profile, involving the expression of interleukin-4, 13, and 15,52 that subsequently evolves in chronic disease. TNF-α plays a fundamental role through activation of cytokine and chemokine expression, expression of endothelial-cell adhesion molecules, protection of synovial fibroblasts, promotion of angiogenesis, suppression of regulatory T cells, and induction of pain.53,54 Similarly, interleukin-6 drives local leukocyte activation and autoantibody production but mediates systemic effects that promote acute-phase responses, anemia, cognitive dysfunction, and lipid-metabolism dysregulation. The central role of these two cytokines has been confirmed by successful therapeutic blockade of membrane and soluble TNF-α and the interleukin-6 receptor in patients with rheumatoid arthritis
Elucidation of the complex intracellular signaling molecules (particularly kinases) that regulate cytokine-receptor–mediated functions may facilitate the development of specific small-molecule inhibitors. Although many intracellular signaling pathways are active in the synovium, clues to those with hierarchical importance have been provided by clinical trials. Positive clinical outcomes in phase 2 studies of the Janus kinase (JAK) 1 and 3 inhibitor tofacitinib implicate JAK pathways that mediate the function of several cytokines, interferons, and growth factors in the pathogenesis of rheumatoid arthritis57,58 (Table 2). Moreover, inhibition of spleen tyrosine kinase by fostamatinib, which is effective in some subgroups of patients, is commensurate with its role in the function of B-cell and Fc receptors.59,60 Other intracellular targets, including phosphatidylinositol 3-kinase, Bruton's tyrosine kinase, and other components of the NF-κB pathway, offer intriguing possibilities for therapeutic strategies. In contrast, despite a strong preclinical rationale, the targeting of p38 mitogen-activated protein kinase has been disappointing in clinical settings, which probably indicates that the molecular signaling network in rheumatoid arthritis has functional redundancy.
Mesenchymal Tissue Responses
The normal synovium contains mesenchymal-derived, fibroblast-like synoviocytes (FLSs) and resident macrophages. In rheumatoid arthritis, the membrane lining is expanded, and FLSs assume a semiautonomous phenotype characterized by anchorage independence, loss of contact inhibition, and the expression of high levels of disease-relevant cytokines and chemokines, adhesion molecules, matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs).61 FLSs thereby contribute directly to local cartilage destruction and the chronicity of synovial inflammation, and they promote a permissive microenvironment that sustains T-cell and B-cell survival and adaptive immune organization.62
The molecular mechanisms that sustain synovial hyperplasia are incompletely understood. The increased proliferative capacity of FLSs is not explanatory. A more likely possibility is altered resistance to apoptosis, which is mediated by diverse pathways, including mutations of the tumor-suppressor gene p5363; expression of stress proteins (e.g., heat-shock protein 70), which foster the survival of FLSs64; and modulation of the function of the endoplasmatic reticulum by synoviolin, an E3 ubiquitin ligase that regulates the balance of cell proliferation and apoptosis.65 Synoviolin negatively regulates p53 expression and its biologic functions. In addition, cytokine-induced activation of the NF-κB pathway in FLSs favors survival after ligation of TNF-α receptor. Methylation and acetylation of cell-cycle regulatory genes and expression of microRNAs may be critical factors.66
Synovial hyperplasia could also reflect increased influx of mesenchymal cells. In a mouse model of arthritis with severe combined immunodeficiency, FLSs were shown to migrate and thereby promote articular involvement.67 A crucial advance has been the elucidation of the molecular pathways that sustain integral membrane structure in rheumatoid arthritis. Cadherin-11 and β-catenin mediate FLS-homotypic interactions that are essential for membrane formation and for subsequent inflammation.68
STRUCTURAL DAMAGE
Cartilage Damage
A hyperplastic synovium is the major contributor to cartilage damage in rheumatoid arthritis. Loss of the normally protective effects of synovium (e.g., reduced expression of lubricin)69 alter the protein-binding characteristics of the cartilage surface, promoting FLS adhesion and invasion. FLS synthesis of MMPs (particularly MMP-1, 3, 8, 13, 14, and 16) promotes disassembly of the type II collagen network, a process that alters glycosaminoglycan content and water retention and leads directly to biomechanical dysfunction. MMP-14 appears to be the predominant MMP expressed by FLSs to degrade the collagenous cartilage matrix.70 Other matrix enzymes (e.g., ADAMTS 5) degrade aggrecan and thus further diminish cartilage integrity.
Endogenous enzyme inhibitors, such as TIMPs, fail to reverse this destructive cascade. Moreover, articular cartilage itself has limited regenerative potential. Chondrocytes physiologically regulate matrix formation and cleavage: under the influence of synovial cytokines (particularly interleukin-1 and 17A) and reactive nitrogen intermediates, cartilage is progressively deprived of chondrocytes, which undergo apoptosis. These processes ultimately lead to the destruction of the surface cartilage and the radiographic appearance of joint-space narrowing.
Bone Erosion
Bone erosion occurs rapidly (affecting 80% of patients within 1 year after diagnosis71) and is associated with prolonged, increased inflammation.72 Synovial cytokines, particularly macrophage colony-stimulating factor and receptor activator of NF-κB ligand (RANKL), promote osteoclast differentiation and invasion of the periosteal surface adjacent to articular cartilage.73 TNF-α and interleukin-1, 6, and potentially 17 amplify osteoclast differentiation and activation.74 Moreover, clinical inhibition of TNF-α, interleukin-6, and RANKL retards erosion in rheumatoid arthritis. Notably, blockade of RANKL acts only on bone, with no effect on inflammation or cartilage degradation.75 Osteoclasts have the acidic enzymatic machinery necessary to destroy mineralized tissues, including mineralized cartilage and subchondral bone; destruction of these tissues leads to deep resorption pits, which are filled by inflammatory tissue.
Mechanical factors predispose particular sites to erosion. Thus, “mechanically vulnerable” sites such as the second and third metacarpals are prone to erosive changes.76 Breach of cortical bone permits synovial access to the bone marrow, which causes inflammation of the bone marrow (osteitis as observed on magnetic resonance imaging), in which T-cell and B-cell aggregates gradually replace marrow fat.77 It is unclear whether these lesions occur in conjunction with synovium-induced erosions or whether osteitis necessarily or independently precedes erosion.78 It is conceivable that rheumatoid arthritis starts in the bone marrow and subsequently involves the synovial membrane.
Eroded periarticular bone shows little evidence of repair in rheumatoid arthritis, unlike bone in other inflammatory arthropathies. Cytokine-induced mediators, such as dickkopf-1 and frizzled-related protein 1, potently inhibit the differentiation of mesenchymal precursors into chondroblasts and osteoblasts (CD271+).79 Mesenchymal stem cells, which have the potential to differentiate into adipocytes, chondrocytes, and osteoblasts, can be detected in the synovium.80,81 However, the biologic characteristics of synovial mesenchymal stem cells, their relationship to FLSs and other stromal cells, and the effect of local inflammation on their activities remain unknown, and an understanding of these factors will crucially inform reparative therapeutic strategies
SYSTEMIC CONSEQUENCES OF RHEUMATOID ARTHRITIS
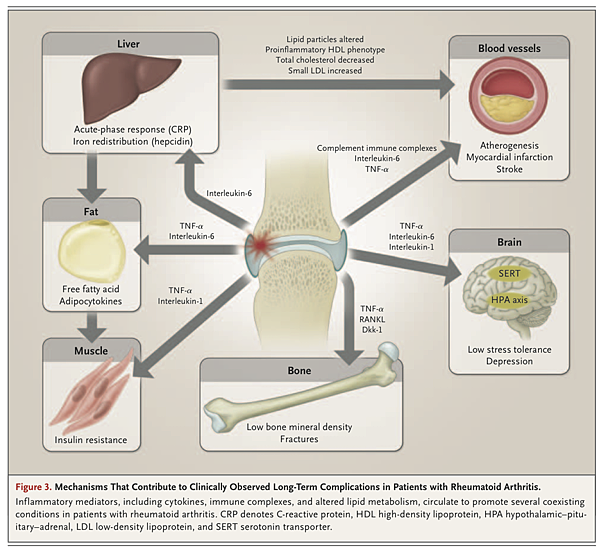
Rheumatoid arthritis is associated with increased rates of cardiovascular illness (standardized mortality rate, approximately 1.5), including myocardial infarction, cerebrovascular events, and heart failure
These increased rates are not explained by traditional risk factors,85,86 use of glucocorticoids or nonsteroidal antiinflammatory drugs, or shared genetic features. Circulating inflammatory pathways that are implicated include cytokines (interleukin-6 and TNF-α), acute-phase reactants, immune complexes, and altered lipid particles (e.g., serum amyloid A–rich HDL) that increase endothelial activation and potentially render atheromatous plaques unstable.87 Increased levels of acute-phase reactants are an independent cardiovascular risk factor in the general population.88 Cytokines also make muscle and adipose tissues insulin-resistant, resulting in an “inflammatory metabolic” syndrome. Moreover, vascular risk is increased early in the course of rheumatoid arthritis, perhaps reflecting subclinical inflammation in the prearticular phase.89,90
Lipid biochemical features are intimately, and reciprocally, linked to inflammation to ensure metabolically efficient host defense. In consequence, active rheumatoid arthritis is associated with reduced serum levels of total, HDL, and LDL cholesterol, which may then be paradoxically elevated by effective therapy.91 Nevertheless, effective therapeutics decrease cardiovascular risk and favorably modify vascular physiology.92-94 Statin drugs also reduce surrogates of vascular risk and inflammatory factors in patients with rheumatoid arthritis, and risk adjustment for statin use in patients with rheumatoid arthritis is now advocated.95
Inflammation in rheumatoid arthritis also affects the brain (fatigue and reduced cognitive function), liver (elevated acute-phase response and anemia of chronic disease), lungs (inflammatory and fibrotic disease), exocrine glands (secondary Sjögren's syndrome), muscles (sarcopenia), and bones (osteoporosis). Osteoporosis affects the axial and appendicular skeleton, with only a modest elevation of the acute-phase response or subclinical inflammation, and probably occurs before the onset of articular disease.96-98 Effective antiinflammatory treatment retards bone loss and suppresses the high rate of systemic bone resorption, as measured with the use of bone-turnover biomarkers.
The risk of lymphoma is increased among patients with rheumatoid arthritis99 and is strongly associated with inflammatory disease activity; sustained disease activity confers the highest risk.100 Clonal selection of B cells, disturbed immune surveillance due to impaired activity of regulatory T cells, and impaired function of natural killer cells are postulated mechanisms.
The higher rates of lung cancer among patients with rheumatoid arthritis than among other persons may be explained in part by the association between smoking and rheumatoid arthritis. However, inflammation increases the risk of lung cancer independently of smoking, perhaps because of the long-known extraarticular effects of rheumatoid arthritis on fibrotic remodeling of interstitial lung tissue.
CONCLUSIONS
The pathogenetic advances described herein have paralleled the introduction of new, effective therapies and remarkable improvement in clinical outcomes. Severe disease manifestations, such as vasculitis, nodule formation, scleritis, and amyloidosis, that are associated with persistent, uncontrolled inflammation have become rare. A rich pipeline of biologic and small-molecule agents, and of potential clinical biomarkers, exists that will add to our therapeutic armamentarium. In time, this should render remission achievable in increasing numbers of patients.
However, much remains to be resolved. We need to understand the factors that lead to loss of tolerance and that cause localization of inflammation in the joint. We need to find ways to promote immunologic resolution or homeostasis and repair of damaged joints. We must elucidate the mechanisms driving the various systemic disorders that contribute substantially to reductions in the quality and length of life. Ultimately, we must strive to develop curative and preventive therapeutics that will transform the notion of rheumatoid arthritis as a chronic disease.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 