美國藥檢局核准新藥Linaclotide膠囊 (145ug) 上市,應用在原發性慢性便秘,以及大腸急躁症 (IBS) 合併便秘症。
The US Food and Drug Administration (FDA) approved the drug linaclotide (Linzess, Ironwood Pharmaceuticals) today for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation (IBS-C) in adults.
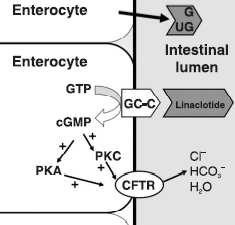
The drug, a capsule intended for once-daily oral administration, is a guanylate cyclase type-C (GC-C) agonist. Linaclotide binds to the GC-C receptor locally in the intestine, with no measurable blood plasma concentrations, resulting in an increase in both intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP), the manufacturer says. Elevations in intracellular cGMP are believed to stimulate secretion of intestinal fluid and accelerate gastrointestinal transit, resulting in increased frequency of bowel movements. Elevations in extracellular cGMP are believed to decrease activity of pain-sensing nerves, which is thought to be responsible for a reduction in intestinal pain, according to nonclinical models.
此藥係屬鳥嘌呤環狀酶C型受體 (GC-C) 協同劑。Linaclotide會與腸道C型受體結合,導致細胞內與細胞外之環狀鳥嘌呤單磷酸基 (cGMP) 增加,而血中此藥濃度卻偵測不到。針對原發性慢性便秘患者,其治療劑量為每日290ug。針對大腸急躁症 (IBS) 合併便秘症患者,其治療劑量為每日145ug~290ug,飯前至少30分鐘服用。
腸道細胞內之環狀鳥嘌呤單磷酸基增加,會刺激腸邑分泌增加,進而增加腸道蠕動。而細胞外之環狀鳥嘌呤單磷酸基增加會減少常到疼痛神經之活性。此藥副作用微腹瀉,16歲以下小孩禁用。
It is the first drug for constipation with this mechanism of action.
Linaclotide's safety and efficacy for the indication of management of IBS-C were established in 2 double-blind studies involving 1604 patients. In those studies, patients were randomly assigned to take 290 μg of the drug or a placebo for at least 12 weeks. Results showed linaclotide to be more effective than placebo in reducing abdominal pain and increasing the number of complete spontaneous bowel movements (CSBMs). These 2 studies in IBS-C are scheduled be published in the October issue of the American Journal of Gastroenterology.
The drug's safety and efficacy for the management of chronic idiopathic constipation were established in 2 further double-blind multicenter trials involving 1272 patients with chronic constipation. These 2 studies were published last year in the New England Journal of Medicine, as reported at the time by Medscape Medical News.
In these studies, patients were randomly assigned to receive either placebo or linaclotide at either 145 or 290 μg once daily.
At week 12, the primary endpoint (of 3 or more CSBMs per week and an increase of 1 or more CSBMs from baseline during at least 9 of the 12 weeks) was reached by significantly more patients receiving linaclotide 145 μg than those receiving placebo. The FDA approval for the indication is only for the 145-μg dose because the improvement in the 290-μg dose group was not more than that seen with 145 μg.
The drug's effects were said to be rapid and observed within the first 24 hours, and to be sustained through 16 weeks.
The authors reporting the chronic constipation in the New England Journal of Medicine speculated that the improvements resulted from factors including increased fluid in the lumen and accelerated intestinal transit.
"Although the improvement in bowel function with linaclotide treatment is most likely a consequence of increased luminal fluid, with an acceleration of intestinal transit, additional mechanisms may contribute to the improvement in abdominal symptoms," they write.
An expert not connected with the studies, Brennan M.R. Spiegel, MD, told Medscape Medical News at the time that linaclotide's improvement in CSBMs was impressive.
"The CSBM rate is probably the most important thing in this condition, so it's important that there were improvements in that," said Dr. Spiegel, associate professor of medicine at the Veterans Affairs Greater Los Angeles Healthcare System and the David Geffen School of Medicine at the University of California, Los Angeles.
Dr. Spiegel also noted that, in addition to increasing luminal fluid, the drug has a unique mechanism that sets it apart from other constipation drugs.
"All of the drugs tend to increase fluid secretion one way or another. For instance, Miralax [MSD Consumer Care] boosts secretion, although passively through osmotic load, whereas linaclotide does it actively by actively pumping chloride into the lumen through the [cystic fibrosis transmembrane conductance regulator] channel and then water gets pumped into the lumen actively," Dr. Spiegel said.
"What is interesting is it works with this guanylate cyclase, which is its unique mechanism of action. But the net result is not really all that unique," he continued.
Dr. Spiegel said he was more enthusiastic about the studies on the drug for IBS-C, which were still underway at the time.
"We have several therapies already available to us for chronic constipation that generally work well, whereas for IBS, it [is] a much different story," Dr. Spiegel said. "In that regard, it looks very promising, not just for constipation but abdominal bloating and pain and a lot of other features, and there aren't many other effective therapies."
However, "[I]if the price point puts it in line with existing therapies, linaclotide could really prove to be one of the first-line agents for difficult to treat chronic constipation," he concluded.
Linaclotide is taken once daily on an empty stomach at least 30 minutes before the first meal of the day.
Its most common adverse effect is diarrhea, and the drug's FDA approval includes a Boxed Warning alerting patients and clinicians that the drug should not be used in patients aged 16 years or younger.
Dr. Spiegel disclosed that he has served on an independent advisory board for Ironwood Pharmaceuticals, but the board was not related to linaclotide.
相關藥物機轉請參考這邊:
圖解藥理學28 laxatives 01





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 