The indication for the oral targeted agent regorafenib (Stivarga, Bayer) has been expanded by the US Food and Drug Administration (FDA); it is now approved for the treatment of gastrointestinal stromal tumors (GIST). Specifically, it is indicated for use in patients with tumors that cannot be surgically removed and no longer respond to imatinib (Gleevec) and sunitinib (Sutent), the 2 other FDA-approved treatments for this disease.
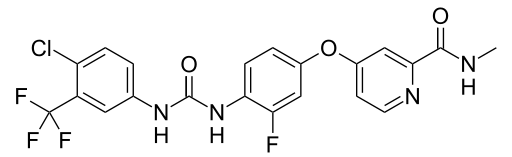
Regorafenib是拜耳大藥廠所研發用於治療轉移性大腸直腸癌患者,Regorafenib是VEGFR2-TIE2 tyrosine kinase,一個多重性的激脢抑制劑,包括血管新生、癌化、基質激脢的抑制劑,在早期的臨床試驗當中顯示出副作用方面是可以接受的,而且有抗腫瘤的效果。
今年國際性腸胃道腫瘤研討會所發表的名為CORRECT臨床試驗,用來評估Regorafenib的對於所有己被証實的標準治療失敗之後其療效以及安全性,這個研究共收錄了760名轉移性大腸直腸癌患者,這些人都是經歷標準治療失敗或者這三個月以內正在接受標準治療。實驗組則是使用Regorafenib 160m g(療程為連續使用三周,爾後休息一週)加上最佳的支持療法,而對照組則使用最佳的支持療法加上安慰劑。病患一直使用至疾病出現惡化、死亡或者不可以接受的毒性。研究的第一要點在於存活時間,第二要點乃在於疾病控制的時間、有效反應的比例、疾病控制的比例、安全性以及生活品質。
整體而言,Regorafenib確實為這些病患帶來有意義的存活效益,大約可以減少死亡的風險約23%。而且疾病控制的時間大約是兩個月,大約是比最佳支持療法約有百分之五十的幫助。而且疾病控制的比例也比對照組多出了快兩倍(44% 比上 15%)。副作用的部份大多可以藉由藥物劑量的調整處理得非常好,正如其過去的臨床實驗所表示。最常見的嚴重副作用是手足反應,大約是17%;其次是15%會有嚴重的疲倦;大約有8%會分別出現第三級的腹瀉、高bilirubine的情形。
研究團隊也發現Regorafenib對於疾病的穩定以及延緩疾病的惡化其效果遠遠優於腫瘤縮小的狀況,而且發現到有一部份的病人對於Regorafenib反應良好的,通常可以保持疾病的持續穩定。他個人也表示自己本身有一位治療Regorafenib已經使用超過十二個月以上的患者。而Regorafenib是目前唯一被証實可以使用在轉移性大腸直腸癌治療上的小分子激脢抑制劑的藥物,這將對未來若治療帶來一線曙光。
Regorafenib is already marketed for use in metastatic colorectal cancer; it was approved for that indication in September 2012.
This is "the third drug approved by the FDA to treat gastrointestinal stromal tumors," said Richard Pazdur, MD, director of the Office of Hematology and Oncology Products in the FDA Center for Drug Evaluation and Research, in a statement. "It provides an important new treatment option for patients with GIST in whom other approved drugs are no longer effective," he explained.
Under the FDA's priority review program, regorafenib was granted orphan product designation because it is intended to treat a rare disease.
"Strong Case" for Use in GIST
The GIST indication was approved on the basis of data from the GRID (GIST– Regorafenib in Progressive Disease) trial (Lancet. 2013;381:295-302). This study, headed by George Demetri, MD, from the Dana-Farber Cancer Institute in Boston, Massachusetts, involved 199 patients with metastatic or unresectable GIST who had progressed after treatment with imatinib and sunitinib. Patients were treated with best supportive care and randomized in a 2:1 ratio to either regorafenib (160 mg daily for 3 weeks followed by a 1-week break) or placebo.
There was a statistically significant improvement in progression-free survival with regorafenib, compared with placebo (4.8 vs 0.9 months; hazard ratio, 0.27; P < .0001).
For the indication metastatic colorectal cancer, regorafenib was approved on the basis of the pivotal CORRECT trial (Lancet. 2013;381:303-312), which was published in the same issue as the GRID trial.
In a comment that accompanied the 2 studies (Lancet. 2013;381:273-275), Tom Waddell, MD, and David Cunningham, MD, FRCP, both from the Royal Marsden Hospital, Sutton, Surrey, United Kingdom, write that there was a "much earlier separation of curves" in the GRID trial than in the CORRECT trial.
However, there was no significant difference in overall survival in the GRID trial, as there was in the CORRECT trial. Drs. Waddell and Cunningham explain that this was "probably attributable to planned extensive crossover to regorafenib" (85% of patients in the placebo group went on to take regorafenib when their disease progressed).
Nevertheless, the editorialists conclude that there is a "strong" case for the routine use of regorafenib in patients who have failed imatinib and sunitinib, whereas the case for using regorafenib in metastatic colorectal cancer is "less compelling."
The most common adverse effects reported with regorafenib are weakness and fatigue, hand-foot syndrome, diarrhea, loss of appetite, high blood pressure, mouth sores, infection, changes in voice volume or quality, pain, weight loss, stomach pain, rash, fever, and nausea.
Serious adverse events are rare and reported in less than 1% of patients. These include liver damage, severe bleeding, blistering and peeling of skin, very high blood pressure requiring emergency treatment, heart attacks, and intestinal perforations.
The GRID and CORRECT trials were sponsored by Bayer, the manufacturer of regorafenib. Several coauthors of the 2 papers report being employed by Bayer or report relationships with pharmaceutical companies, as detailed in the papers. Dr. Demetri reports serving as a consultant to Novartis, Pfizer, Lilly, Infinity, GlaxoSmithKline, Plexxikon, Koltan, and Blueprint Medicines. Dr. Waddell has disclosed no relevant financial relationships. Dr. Cunningham's research unit has received funding from Amgen, Roche, Celgene, AstraZeneca, Merck-Serono, and sanofi-aventis.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 