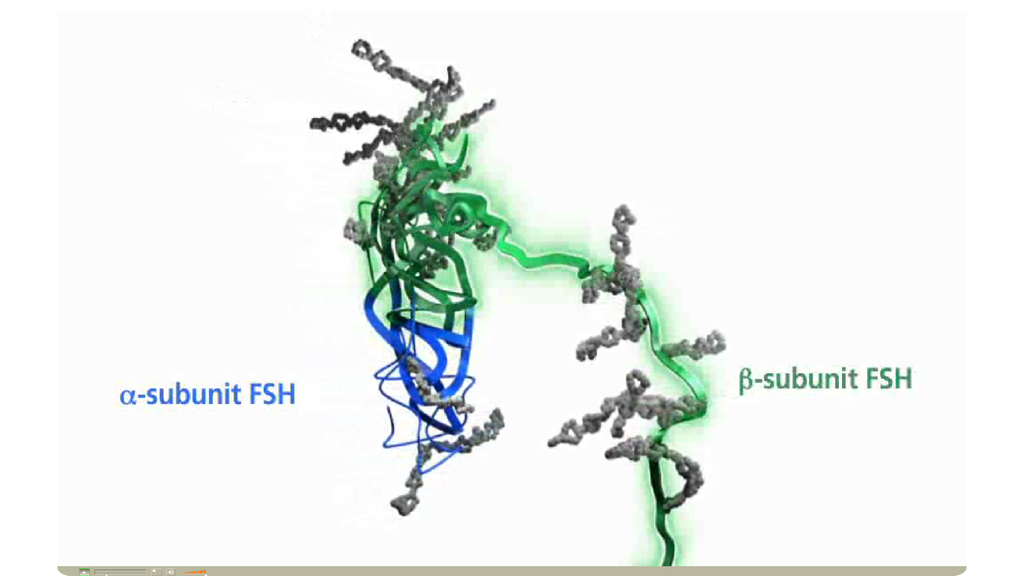
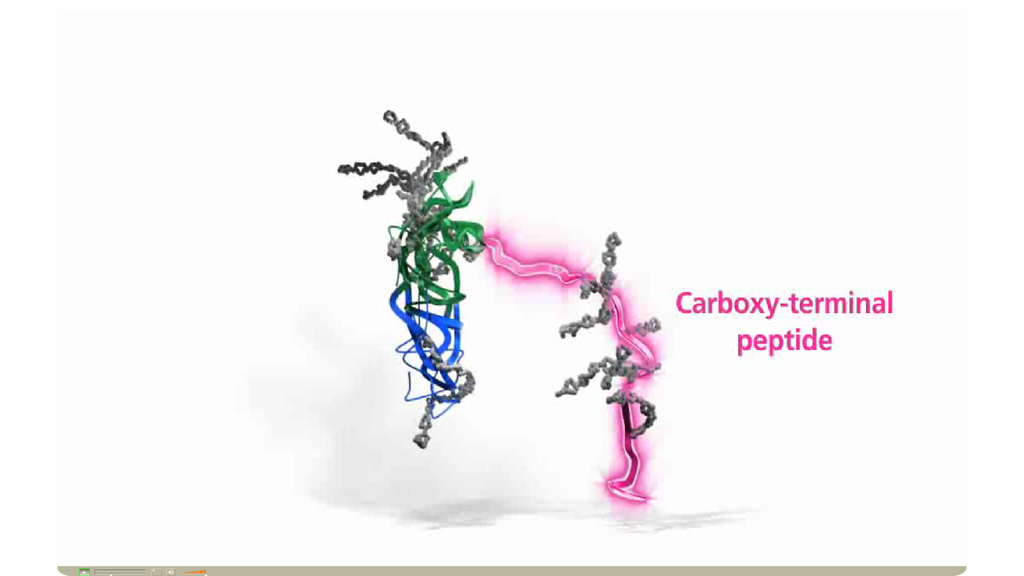
Corifollitropin alfa (Elonva®)的結構異於recombinant FSH。Corifollitropin alfa, the active ingredient of ELONVA®, is a novel fully human recombinant fertility hormone composed of the α subunit of human FSH and the β subunit of human FSH fused with the carboxy terminal peptide of the human chorionic gonadotropin (hCG)
除原本人類促濾泡成熟激素(FSH)分子結構中的α次單位外,將人類絨毛膜性腺激素(hCG)之β次單位的尾端carboxy terminal peptide加入人類的FSH的β鏈中,因此有類似hCG半衰期較長的特性。
機轉:
作用在 FSH receptor,但是沒有intrinsic LH/hCG活性。
可以在這個網頁看影片:
點我
在藥物動力學分析上,Corifollitropin alfa(Elonva®)的半衰期為FSH的1.5至2倍,約為65小時。因此,Corifollitropin alfa(Elonva®)可提供較長時間並且足夠之FSH血中濃度來刺激濾泡的生長。臨床使用上,施打一劑後,可維持7天藥效,一星期後藥效不足時可每天再追加短效型rFSH一至三劑至濾泡成熟。
但是像這樣促進生育的藥物,懷孕分級要怎樣算呢?
首先,當然是要參考一下仿單
我相信你也發現了:他沒寫!
所以這時候我們就必須找一下台灣最容易查到的大型資料庫Micromedex:
不過遺憾的,他只寫了unknown
別放棄,因為我們可以從他的姊妹品Gonal-F (follitropin alfa)略之一二:
A)
Teratogenicity/Effects in Pregnancy
1) U.S. Food and Drug Administration's Pregnancy Category: Category X (All Trimesters)
a) Studies in animals or human beings have demonstrated fetal abnormalities or there is evidence of fetal risk based on human experience or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant.
2) Australian Drug Evaluation Committee's (ADEC) Category: B3
a) Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans.
3) Crosses Placenta: Unknown
4) Literature Reports
a) In vitro and in vivo mutagenicity studies revealed that follitropin alfa does not possess mutagenic or clastogenic activity. No significant increase in chromosomal aberrations was detected with follitropin alfa .
B)
1) Micromedex Lactation Rating: Infant risk cannot be ruled out.
a) Available evidence and/or expert consensus is inconclusive or is inadequate for determining infant risk when used during breastfeeding. Weigh the potential benefits of drug treatment against potential risks before prescribing this drug during breastfeeding.
2) Clinical Management
a) Follitropin alfa should not be used during lactation
結果更怪的答案出現了:懷孕分級出現兩種版本(不是指美國和澳洲),而且還差距頗大。
你可以先參考兩種國家的分級法:
為什麼會差異如此大呢?所以這件事情,我就直接打電話去原廠問了。
原廠的答案是這樣的:
- 因為這是屬於歐系藥,歐系藥對於沒有做過實驗的藥品不會寫上分級,所以這個藥物就沒有分級。
- 但是因為輸出到其他國家,所以就必須依照其他國家的法規來劃分懷孕分級,因為從來沒有做過孕婦實驗,所以美國那邊決定列為X級。澳洲那邊是比照英國的方式,英國那邊認為hCG及FSH沒有致畸胎性,加上這個藥物在發現懷孕時就會立即停止使用了,所以是相對安全的,因此列為B3等級。
當然,為了慎重起見,我去找了澳洲那邊的報告給大家參考:











 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 