納美人?

Neurologists and other physicians using the antiseizure drug Potiga (ezogabine, Valeant Pharmaceuticals) as an adjunct treatment in patients with partial-onset epilepsy should be on the lookout for blue skin discoloration and retinal abnormalities and report these adverse effects should they occur.
The Food and Drug Administration (FDA) has issued a warning that Potiga can cause pigment changes and eye abnormalities. According to a government press release, it's not known at this time whether these changes are reversible. The FDA said it is working with the manufacturer to gather and evaluate all available information to better understand these developments and will update the public when more information is available.
The FDA recommends that all patients taking Potiga have a baseline eye examination and periodic eye examinations that should include visual acuity testing and dilated fundus photography. Periodic examinations may include fluorescein angiography, ocular coherence tomography, perimetry, and electroretinography.
Lips and Nail Beds
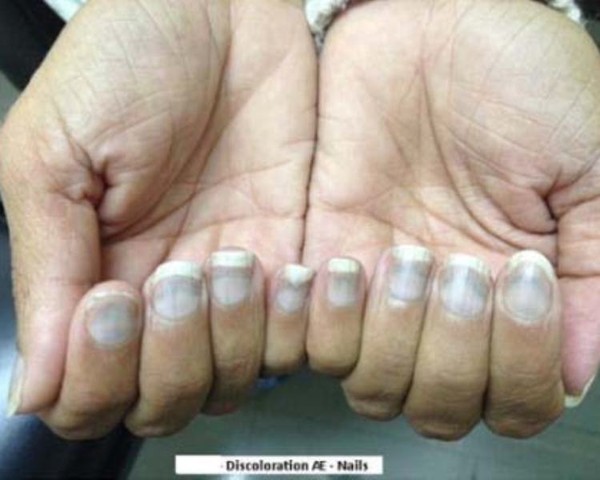
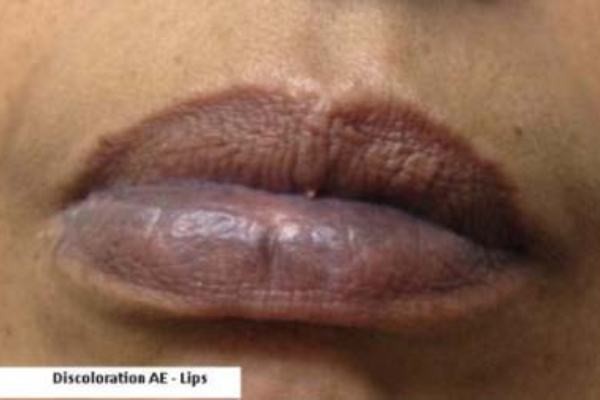
In the reported cases, the skin discoloration appeared as blue pigmentation, predominantly on or around the lips or in the nail beds of the fingers or toes, but more widespread involvement of the face and legs has also been reported. Scleral and conjunctival discoloration, on the white of the eye and inside eyelids, has been observed as well.
The skin discoloration generally occurred after 4 years of treatment with Potiga but has appeared sooner in some patients. In certain cases, retinal abnormalities have been observed in the absence of skin discoloration.
Patients taking Potiga who develop changes in their vision or discoloration of their skin, including their lips and nail beds, should be advised to immediately contact their doctor.
They should also be told not to stop taking Potiga without first conferring with their healthcare professional. Suddenly ceasing such treatment can cause serious and life-threatening medical problems, such as recurrence of seizures.
Potiga is a potassium channel opener indicated as an adjunct treatment of partial-onset seizures in patients aged 18 years or older. It was approved by the FDA in 2011.
Previously identified adverse effects include urinary retention, neuropsychiatric symptoms, suicidal thinking or behavior, dizziness and somnolence, QT interval effect, and withdrawal seizures.
Adverse reactions or quality problems experienced with the use of Potiga may be reported to MedWatch, the FDA's safety information and adverse event reporting program, by telephone at 1-800-FDA-1088; by fax at 1-800-FDA-0178; online at https://www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm; with postage-paid FDA form 3500, available at http://www.fda.gov/MedWatch/getforms.htm; or by mail to MedWatch, 5600 Fishers Lane, Rockville, Maryland 20852-9787.
當然不是納美人那種啦
最後,這邊有小整理:





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 