N Engl J Med 2014; 370:1227-1236March 27, 2014DOI: 10.1056/NEJMra1304623
Critically ill patients requiring vital organ support in the intensive care unit (ICU) commonly have anorexia and may be unable to feed volitionally by mouth for periods ranging from days to months. Unless such patients are provided with macronutrients in the form of enteral or parenteral nutrition, they accumulate an energy deficit that rapidly reaches proportions that contribute to lean-tissue wasting and that are associated with adverse outcomes.1 The catabolic response to acute critical illness is much more pronounced than that evoked by fasting in healthy persons, since the energy deficit in acutely ill patients is often superimposed on immobilization and pronounced inflammatory and endocrine stress responses. Severe skeletal-muscle wasting and weakness occurring during critical illness are associated with a prolonged need for mechanical ventilation and rehabilitation.2
In many studies, the degree of energy deficit accumulating in critically ill patients is strongly associated with the duration of stay in the ICU, which, in turn, is associated with an increased incidence of infectious complications and risk of death.1 Until recently, however, the causality of these associations remained unclear, since the majority of studies that formed the basis of published recommendations for feeding ICU patients were either observational or small interventional studies.3,4 Recently, the field of critical care nutrition has been revived by the findings of several randomized, controlled trials, which have opened a new debate on nutritional practice in the ICU.
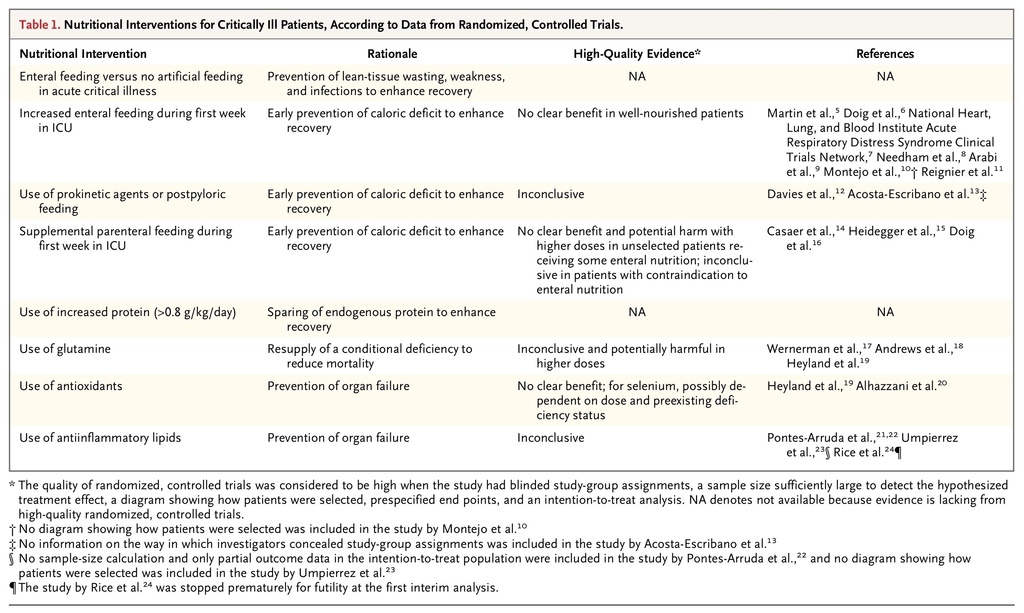
For this review, we focus on evidence from randomized, controlled studies that met the following criteria: study-group assignments were made in a blinded fashion, the sample size was sufficiently large to detect a prespecified treatment effect, there was a clear delineation of the way in which patients were selected and followed, there was a statistical analysis plan with end points defined a priori, and there was an intention-to-treat analysis with adequate handling of competing risks (Table 1
TABLE 1
Nutritional Interventions for Critically Ill Patients, According to Data from Randomized, Controlled Trials.
). In this review, we integrate this newer evidence with older high-level evidence to provide suggestions for feeding during the acute phase of critical illness. In cases in which such evidence does not exist, we identify areas of uncertainty that require further investigation.
ENTERAL NUTRITION
Timing of Initiation
Anorexia is part of the acute physiologic response to severe illness that can be either adaptive or maladaptive. Studies in animals and humans have shown a trophic effect of enteral nutrients on the integrity of the gut mucosa, a finding that has provided the rationale for instituting enteral nutrition early during critical illness. In observational studies, patients in the ICU who were fed early through the enteral route have had a better outcome than those who were not.25 However, the inability to provide enteral nutrition early may be a marker of the severity of illness (i.e., patients who can be fed enterally are less ill than those who cannot) rather than a mediator of complications and poor outcomes.
Hence, the first question to address is whether data from methodologically sound, randomized, controlled trials support the initiation of enteral feeding early in the acute phase of critical illness. A meta-analysis of six small trials involving a total of 234 patients in the ICU showed a survival benefit with the immediate initiation of enteral nutrition, as compared with delayed initiation.26 Unfortunately, the quality of the individual studies in this meta-analysis was poor. For example, in three of the studies, the comparator group received parenteral nutrients within 24 hours after ICU admission, a factor that made the interpretation of the results difficult.27 The rationale for the early initiation of enteral nutrition rather than parenteral nutrition is the lower risk of infection with enteral nutrition that was observed in older randomized, controlled trials that were conducted in an era in which investigators may have underestimated the harm of pronounced hyperglycemia and overfeeding in critically ill patients.28 However, large, high-quality, randomized, controlled trials supporting an outcome benefit with early enteral nutrition versus delayed nutrition during the acute phase of critical illness have not been performed.
Estimation of Energy Requirements
The energy requirements of critically ill patients continue to be debated.29 Such requirements are often estimated with the use of characteristics of the patients before the onset of illness.29 Other observers argue that energy requirements differ per patient and per day in the ICU and thus should be individually estimated on a daily basis from measurements of oxygen consumption and carbon dioxide production, as determined with the use of indirect calorimetry.29 However, this method is often technically difficult or impossible to perform in critically ill patients.29
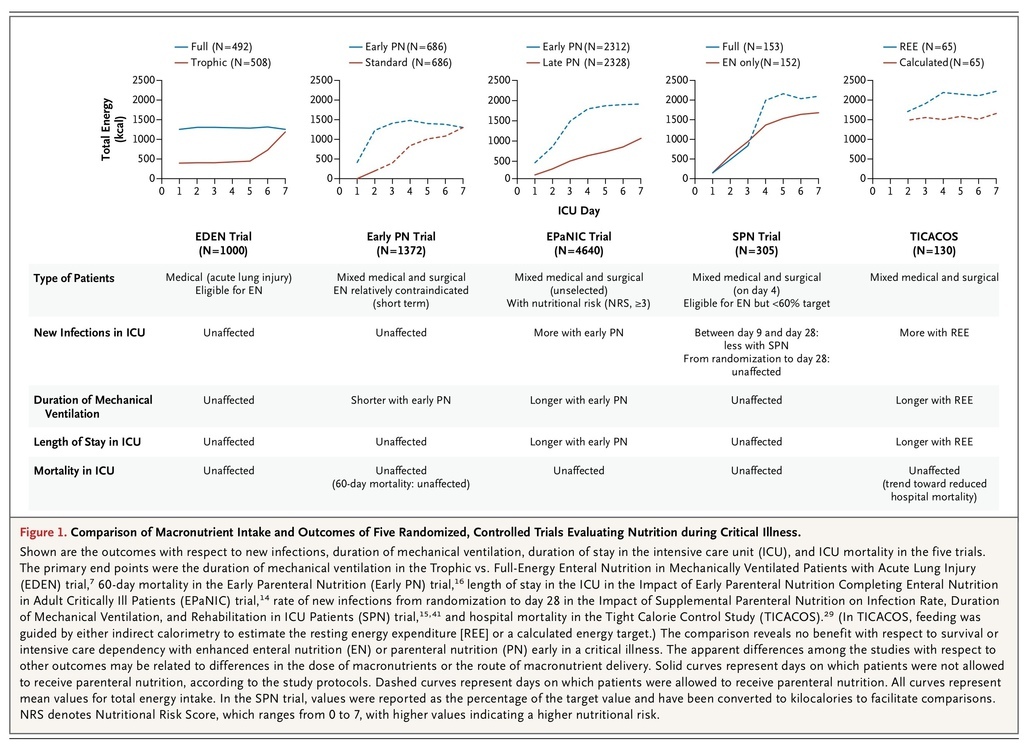
In the Tight Calorie Control Study (TICACOS) involving 130 patients, those who were undergoing mechanical ventilation in an ICU and who received nutrition that was guided by indirect calorimetry to estimate the resting energy expenditure received more nutrition than did control patients, who were fed with a calculated energy target. The intervention led to a trend toward reduced hospital mortality but a significant increase in infections and in the length of stay in the ICU30 (Figure 1
FIGURE 1
Comparison of Macronutrient Intake and Outcomes of Five Randomized, Controlled Trials Evaluating Nutrition during Critical Illness.
).
Because of interruptions in feeding for a variety of reasons and delayed gastric emptying, patients often receive less than the prescribed amount of enteral nutrition. Failure to deliver the prescribed nutrition has been considered to be one of the reasons that the use of enteral nutrition has not improved the outcome in critically ill patients. This hypothesis was supported by a small, randomized, controlled trial involving patients with traumatic brain injury, which showed that delivering enteral nutrition to reach an estimated energy target immediately after ICU admission, rather than to reach gradually increasing targets over the first week in the ICU, resulted in a reduced rate of infection.31
To address these issues, approaches were successfully developed to deliver larger amounts of enteral nutrition earlier during critical illness.5 Two large, cluster-randomized trials5,6 enrolling 462 and 1118 patients, respectively, investigated the effect of such protocols on clinical outcomes. In the two studies, the protocols increased the amount of nutrition delivered. In the smaller study, implementation of the protocol resulted in a decreased length of hospital stay and a nonsignificant reduction in hospital mortality. In the larger study, in which participating centers were not allowed to cross over, implementation of the protocol resulted in an earlier initiation of feeding and an increased attainment of caloric goals, but this did not provide any benefit in terms of either mortality or length of stay in the ICU or hospital.6
In the more recent Trophic vs. Full-Energy Enteral Nutrition in Mechanically Ventilated Patients with Acute Lung Injury (EDEN) trial, investigators addressed the question of how much enteral nutrition should be administered early during critical illness7 (Figure 1). In this study, 1000 relatively young and well-nourished patients with acute lung injury who were in the ICU and who were considered to be eligible for enteral nutrition were randomly assigned to receive either just a small amount of (trophic) enteral feeding for 1 week in the ICU or full enteral feeding from the time of admission onward. The first 272 patients who were enrolled underwent concomitant randomization in the OMEGA trial, which investigated the effect of n–3 fatty acids in patients with acute lung injury. In accordance with the protocol of that trial, patients received an additional amount of lipids or an isocaloric protein dose.24 The EDEN trial was a large study with a high methodologic standard. Although the patients in the group receiving trophic feeding accumulated a substantially greater nutritional deficit than did the group receiving full enteral feeding for 1 week, there was no between-group difference in acute or long-term functional outcomes.7,8 These results are consistent with those of a smaller randomized, controlled trial involving 240 patients, in which patients with a variety of illnesses who were in the ICU were assigned either to an approach that allowed underfeeding or to one that targeted full feeding. Patients who were assigned to the approach that allowed underfeeding received fewer calories but had outcomes that were at least as good as those in patients assigned to early full feeding.9
Gastric Residual Volume
Among patients in the ICU, gastric emptying is often slow or impaired, which can result in large gastric residual volumes with enteral feeding. Since regurgitation of gastric content can lead to aspiration pneumonia, enteral feeding is often discontinued in patients who are found to have large gastric residual volumes. For this reason, there is a longstanding controversy about whether the presence of large gastric residues is acceptable. Two recent randomized, controlled trials addressed this question. The Gastric Residual Volume during Enteral Nutrition in ICU Patients (REGANE) trial (involving 329 patients) showed that gastric residual volumes up to 500 ml could be safely tolerated.10 The Effect of Not Monitoring Residual Gastric Volume on the Risk of Ventilator-Associated Pneumonia in Adults Receiving Mechanical Ventilation and Early Enteral Feeding (NUTRIREA 1) trial (involving 449 patients) showed that the omission of the measurement of gastric residual volumes did not increase the incidence of aspiration or related complications.11 Interestingly, the two studies showed that allowing large gastric residual volumes increased the amount of enteral feeding that was administered early during critical illness but did not affect clinical outcomes.
The administration of prokinetic agents such as metoclopramide or erythromycin has been advocated to improve gastric emptying. Erythromycin may be more effective than metoclopramide, but benefits with respect to clinical outcome have not been shown.32,33 Prokinetic-resistant impaired gastric emptying is sometimes considered to be an indication to bypass the stomach and to deliver nutrition directly beyond the pylorus. Although postpyloric feeding may allow increased amounts of enteral nutrition to be given early, findings from randomized, controlled trials are inconclusive regarding the effect on clinical outcome. 12,13
PARENTERAL FEEDING
When clinicians rely solely on the enteral route to feed patients in the ICU, the numbers of calories that are administered often do not meet the calculated targets. This discrepancy can be due to side effects associated with enteral feeding or to the lack of a functional gastrointestinal tract. The clinical question remains whether parenteral nutrition should be initiated in such patients and, if so, when.
The findings of observational and small intervention studies have been inconclusive.28,34 Although European guidelines have recommended the early initiation (within 48 hours after admission to the ICU) of parenteral nutrition so that the accumulating nutritional deficit is prevented as soon as possible, American and Canadian guidelines have advised allowing hypocaloric enteral nutrition for 1 week in well-nourished patients before considering parenteral nutrition.3,4 The latter advice was based on the observation of complications (e.g., liver-function abnormalities, hyperglycemia, hypertriglyceridemia, and infections) associated with parenteral nutrition and overfeeding reported in older studies.28,35-37
In a large, randomized, controlled trial — the Impact of Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients (EPaNIC) trial14 — patients in the two groups received tight glucose control (target blood glucose level, 80 to 110 mg per deciliter [4.4 to 6.1 mmol per liter]) and parenteral trace elements and vitamins, which were administered until the initiation of adequate enteral feeding (Figure 1). Patients who received early parenteral nutrition to supplement insufficient enteral nutrition were given parenteral glucose for 2 days (approximately 100 g on day 1 and 180 g on day 2), which was followed from day 3 onward by the administration of commercially available all-in-one parenteral nutrition preparations delivering on average 40 g of protein per liter per day. The study enrolled 4640 critically ill patients with an average score of 23 on the Acute Physiology and Chronic Health Evaluation II (APACHE II) (on which scores range from 0 to 71, with higher scores indicating more severe disease) who were admitted after cardiac surgery, medical complications after surgery, multiple or cerebral trauma, sepsis, or other life-threatening emergencies. After 1 week in the ICU, the delivered enteral nutrition in the two study groups was approximately 20% of the estimated energy requirements. Unexpectedly, patients who received insufficient enteral nutrition had an earlier live discharge from the ICU and hospital, a lower incidence of new ICU infections and of ICU-acquired weakness,38 and a lower duration of vital-organ support than did patients receiving insufficient enteral nutrition supplemented with parenteral nutrition. 14 There were substantial cost savings in the group not receiving the parenteral nutrition, which were explained largely by a reduced need for antibacterial and antifungal drugs.39 Results were consistent regardless of the type or severity of illness.14,40
In a second trial, the Impact of Supplemental Parenteral Nutrition on Infection Rate, Duration of Mechanical Ventilation, and Rehabilitation in ICU Patients (SPN) study,15 investigators addressed the pragmatic question of how to treat patients who are eligible to be fed enterally but who cannot tolerate full enteral feeding after 3 days (12% of admissions in the SPN study) (Figure 1). The study enrolled 305 patients who were not yet receiving 60% of the energy goal on the fourth day in the ICU, a target that was determined on the basis of indirect calorimetry performed in 65% of the patients. Patients were randomly assigned to receive supplemental parenteral nutrition or enteral nutrition alone from day 4 in the ICU to day 8. The mean (±SD) between-group difference in energy intake over the 4 intervention days was about 25% of the target (103±18% vs. 77±27%). The incidence of infections between day 9 and day 28 was lower among patients assigned to the parenteral-nutrition group. However, the rate of infection during the first 28 days of the ICU stay, which was the primary end point of the study, was unaffected.15,41 The randomized intervention had no significant effect on other clinical end points. In contrast to earlier trials, the administration of parenteral nutrition was not associated with an increased risk of infection. However, it was also not associated with a net clinical benefit.
In a third trial, the Early Parenteral Nutrition study,16 investigators addressed another pragmatic question: should parenteral nutrition be administered early to the subgroup of critically ill patients who have a relative contraindication to early enteral nutrition? In this study, 1372 patients from 31 ICUs were assigned to receive either early parenteral nutrition (as an all-in-one preparation containing 33 g of protein per liter) within 24 hours after ICU admission or standard therapy at the discretion of the treating physician (Figure 1). Patients were enrolled an average of 13.8 hours after ICU admission, and early parenteral nutrition was started within an hour after enrollment. In the standard-therapy group, 253 of 686 patients (36.9%) received parenteral nutrition during the first few days in the ICU, with 27.1% of them receiving parenteral nutrition after a mean of 1.99 days and another 7.0% receiving supplemental parenteral nutrition with enteral nutrition after a mean of 5.58 days. The protocol advised study physicians to prescribe parenteral trace elements and vitamins only for the group receiving early parenteral nutrition. There was no significant between-group difference in the primary end point, 60-day mortality, but the duration of mechanical ventilation (a tertiary outcome) was shorter in the group receiving early parenteral nutrition.16
It remains unknown whether early parenteral nutrition is beneficial for patients who have an absolute and more prolonged contraindication to enteral nutrition. Since the avoidance of parenteral feeding results in prolonged fasting, such patients are often excluded from studies. A meta-analysis of seven randomized, controlled trials published between 1981 and 1994 (involving a total of 798 patients) showed that parenteral nutrition, as compared with no feeding, was associated with a higher rate of infection.42 In a post hoc subgroup analysis of the EPaNIC trial, 517 patients who were admitted to the ICU with a surgical contraindication for enteral feeding had fewer infections and an increased likelihood of earlier live discharge from the ICU if they were not assigned to receive early parenteral nutrition.14 For these patients, starvation was tolerated for 1 week in the ICU and resulted in improved clinical outcomes.14 Thus, the most effective time at which the initiation of parenteral nutrition can produce a clear clinical benefit during critical illness remains unclear.
SELECTION OF MACRONUTRIENTS
Amino Acids
Another controversial topic is the preferred amino acid content of enteral and parenteral preparations. Gluconeogenesis, which uses amino acids generated from the breakdown of skeletal muscle, cannot be fully suppressed by exogenous glucose during critical illness. This phenomenon can be explained, in part, by the complex stress response, which causes hepatic insulin resistance. It was therefore inferred that the administration of exogenous protein could induce a protein-sparing effect in critically ill patients through the stimulation of protein synthesis. However, analyses of the association between protein intake and outcome have shown conflicting results.40,43 Only one randomized, controlled trial, involving 50 patients who were being treated with renal-replacement therapy, has studied the effect of increasing the protein dose. In this highly selected population, increased amounts of protein altered the calculated nitrogen balance but not the clinical outcome.44 Hence, the most effective protein-to-energy ratio for critically ill patients remains unknown.
Glutamine is the most abundant nonessential free amino acid. It is synthesized predominantly in skeletal muscle; low glutamine levels have been associated with a poor outcome in critical illness. Low glutamine levels were considered to be the consequence of muscle wasting, since with the loss of muscle mass, the production of glutamine may not match increased glutamine requirements of immune cells, enterocytes, and hepatocytes. Thus, glutamine was labeled a “conditionally essential” amino acid during critical illness, which led to the hypothesis that glutamine supplementation would improve outcomes. A meta-analysis of the early randomized, controlled trials involving a total of 485 patients suggested that glutamine supplementation might decrease the risk of infection, the length of stay in the hospital, and the risk of death.45 A Scandinavian trial that was stopped early because of slow recruitment showed no difference in the rate of death or organ dysfunction with glutamine supplementation.17
In two recent high-quality, randomized, controlled trials, investigators studied the effects of two doses of glutamine in critically ill patients. In the Scottish Intensive Care Glutamine or Selenium Evaluative Trial (SIGNET),18 involving 500 patients, investigators evaluated the effects of a glutamine dose of 0.1 to 0.2 g per kilogram per day, whereas in the Reducing Deaths Due to Oxidative Stress (REDOXS) trial,19 involving 1223 patients, investigators evaluated a glutamine dose of 0.6 to 0.8 g per kilogram per day. The SIGNET trial showed no benefit of low-dose glutamine administered parenterally to patients receiving parenteral feeding.18 The REDOXS trial showed an absolute increase of 6.5 percentage points in the rate of death at 6 months among patients with organ failure who received early high-dose parenteral nutrition plus enteral glutamine treatment.19 These results challenge the concept of conditional glutamine deficiency, and robust and consistent trial data do not support routine glutamine supplementation.
Arginine supplementation in the postoperative period may decrease the rate of infectious complications and the length of stay in the hospital. However, current evidence does not support its use during critical illness.46
Lipids
The type of lipid used in nutritional formulations may affect inflammation. The n–3 fatty acids that are present in fish oil have been shown to have antiinflammatory effects, n–9 fatty acids that are present in olive oil have a more neutral immune effect, and n–6 fatty acids in soybean oil are proinflammatory. 47
On the basis of low circulating levels of n–3 fatty acids in patients with acute lung injury and the proinflammatory properties of n–6 fatty acids, the lipid profile of nutrients for such patients was hypothesized to contribute to the development or worsening of acute lung injury. In three pioneering studies involving a total of 411 patients, a modified enteral-feeding program with increased ratios of n–3 fatty acids to n–6 fatty acids resulted in reduced rates of death and new organ failure, along with reduced durations of stay in the ICU, as compared with a feeding program that was based on lipids present in corn or canola oil.21 A later trial showed similar clinical effects in patients with sepsis.22 The most recent and largest trial, the OMEGA study, was stopped prematurely for futility when it showed no benefit with the enteral administration of n–3 fatty acids plus antioxidant supplements in 272 patients.31 In addition, this study showed fewer ventilator-free days and longer stays in the ICU among patients in the n–3 group. Parenteral preparations based on fish oil have not been shown to benefit patients in the ICU,48 and the use of olive oil (an n–9 fatty acid), as compared with soybean oil, did not affect either inflammation or outcomes in a trial involving 100 patients in the ICU.23 Currently, the lack of high-quality evidence precludes any recommendation on the use of specific lipids in critically ill patients.
SELECTION OF MICRONUTRIENTS
Micronutrients (consisting of trace elements, vitamins, and electrolytes) are administered to critically ill patients to prevent deficiencies and associated complications. After depletion of micronutrient stores during starvation, the reinitiation of nutrition can result in a refeeding syndrome, most typically unmasking deficiencies in thiamine, potassium, and phosphate that may cause potentially fatal complications, such as cardiac failure, lactic acidosis, arrhythmia, and respiratory failure.49 As a result, the routine administration of intravenous micronutrients is justified during the acute phase of critical illness until full enteral intake is reached.
The administration of pharmacologic doses of trace elements (selenium, copper, manganese, zinc, and iron) and vitamins (E, C, and beta carotene) has been proposed to reduce oxidative cellular damage and organ failure in critically ill patients. Despite encouraging results from the meta-analysis of early studies,50 an adequately powered, high-quality, randomized, controlled trial showed no such benefit.19 Selenium supplementation may be beneficial in populations in which selenium deficiency is prevalent, and the potential for benefit is supported by a recent meta-analysis.20 Thus, it is possible that factors such as the dose, the presence or absence of deficiency before the onset of illness, and the type of critical illness may determine the benefit of the intervention.20
CONCLUSIONS
Recent methodologically sound and adequately powered randomized, controlled trials have not generated unequivocal evidence that feeding protocols targeting full-replacement nutrition early in the course of critical illness result in clinical benefits. The findings of the EPaNIC and EDEN trials raise concern that targeting full-replacement feeding early in critical illness does not provide benefit and may cause harm in some populations or settings. The Early Parenteral Nutrition and Supplemental Parenteral Nutrition trials suggest that the use of parenteral nutrition in itself may not increase the risk of infectious complications. These new insights limit the number of nutritional interventions that can be confidently recommended for daily critical care practice (Table 2
TABLE 2
Recommendations for Clinical Nutritional Practice in the ICU and for Future Research.
). In light of the new evidence from trials that included few if any severely malnourished patients, it seems reasonable to initiate some gastric feeding, while also providing micronutrients, once the patient's condition is stabilized and to allow hypocaloric macronutrient intake during the first week of critical illness. Whether patients with preexisting malnutrition should be treated differently is uncertain.14,42
It does not appear to be desirable to interfere with the early catabolic response to critical illness, either with macronutrients or, as shown previously,51 with anabolic hormones. Although the unfavorable effect of large amounts of macronutrients and growth factors during acute illness might be explained by their suppressive effects on pathways of cell-damage removal that recycle substrates from clearing debris,38 more research is needed to unravel the exact underlying mechanisms. In addition, research focusing on biomarkers and on scoring systems should aim to identify patients who are able to effectively use macronutrients for recovery and thus are likely to benefit from more aggressive earlier nutrition.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 