FDA核准第一個可吸收之纖維素封劑(TachoSil)用於心血管手術
By Yael Waknine
Medscape Medical News
April 6, 2010 — The US Food and Drug Administration (FDA) has approved the first absorbable fibrin sealant patch for use in cardiovascular surgery (TachoSil, Nycomed Austria GmbH) to prevent mild and moderate bleeding from small blood vessels when standard surgical techniques are ineffective or impractical.
The ready-to-use biodegradable product consists of a sponge made from equine tendons and coated with a dry layer of the human coagulation factors fibrinogen and thrombin. When applied to a wound, the patch mimics the final steps of the natural blood clotting process, creating a hemostatic fibrin clot within 3 to 5 minutes that dissolves within 4 to 6 months.
FDA's action was based on data from a study (n = 119) showing that use of the fibrin sealant patch was significantly more effective than standard hemostatic fleece for achieving hemostasis in cardiovascular surgery patients with persistent hemorrhage (74.6% vs 33.3%).
"This approval provides an additional tool for surgeons to help control mild and moderate bleeding from blood vessels during cardiovascular surgery when standard surgical techniques are ineffective or impractical," said Karen Midthun, MD, acting director of the FDA's Center for Biologics Evaluation and Research, in an agency news release.
Although adverse events reported in the study did not differ significantly between treatment groups, allergic type hypersensitivity reactions may occur if the fibrin sealant patch is applied repeatedly or used in patients with known hypersensitivity to its components. Thromboembolic complications are also possible with intravascular application.
Because the fibrin sealant patch is derived from human and equine products, the potential for transmission of infective agents cannot be totally excluded despite screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps to inactivate/remove viruses.
作者:Yael Waknine
出處:WebMD醫學新聞
【24drs.com】2010 — 美國食品藥物管理局(FDA)核准第一個可吸收之纖維素封劑貼片(商品名TachoSil,Nycomed Austria GmbH公司)用於心血管手術,以預防標準手術技術無效或無法使用時的輕微到中度出血。
這個即拆即用的可分解產品,包括一個由馬腱製成的海綿,包覆一層乾燥的人類凝血因子纖維素原和凝血原,用到傷口上時,這個貼片會模擬自然凝血過程的最後步驟,在3-5分鐘內建立一個止血纖維素塊,在4-6個月內崩解。
FDA的核准是根據一篇有119名研究對象的研究資料,該研究顯示,持續出血之心血管手術病患使用纖維素封劑貼片,比標準止血織物更有效達到止血(74.6% vs 33.3%)。
FDA生物製劑評估與研究中心行動主任Karen Midthun醫師在該局的新聞稿中表示,這個核准提供一個新工具給外科醫師,當進行心血管手術而標準手術技術無效或無法使用時,可幫助控制輕微到中度的血管出血。
雖然兩個治療組之間的不良反應報告並無顯著差異,若重複使用纖維素封劑、或者用在已知對其成分過敏的病患時,會發生過敏型的過敏反應,於血管內使用時也可能發生栓塞併發症。
因為纖維素封劑貼片是衍生自人類和馬的產品,儘管有對個別來源進行特定感染標記之篩檢,並且納入有效的製程達到去活化/移除病毒,仍無法完全排除可能會傳染感染源的可能性。
Tachosil簡介:
包裝就是這樣:

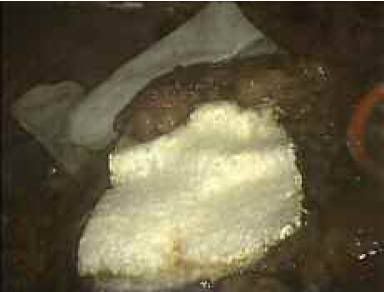
外傷敷料
有各種的大小

可以近看一下,其實就是纖維組織

使用時就是直接添加敷料在上口部位,之後就會和你的身體組織吸收結合。
之前也有介紹過類似的產品:





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 