Author Information
Department of Leukemia, The University of Texas, M.D. Anderson Cancer Center, Houston, Texas, USA
Correspondence: Dr Elias Jabbour, Department of Leukemia, The University of Texas, M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030, USA.
1. Myelodysplastic Syndromes (MDS)
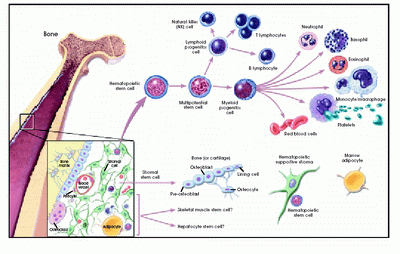
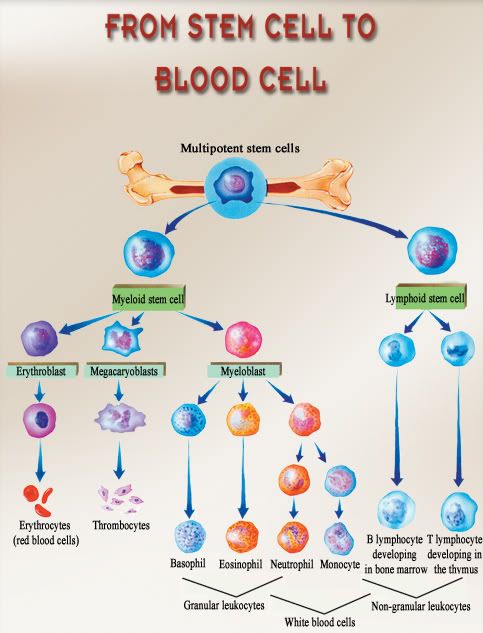
Myelodysplastic syndromes (MDS) are a group of heterogeneous haematopoietic stem cell disorders characterized by peripheral blood cytopenias, bone marrow hypercellularity and dysplasia affecting one or more of the haematopoietic stem cell lines. Patients with MDS are at risk for transformation to acute myeloid leukaemia (AML), and this risk increases with more advanced disease. MDS primarily occurs in people over 60 years of age and many patients suffer from complications related to cytopenias. Therefore, MDS is associated with substantial morbidity and mortality.[1-4]
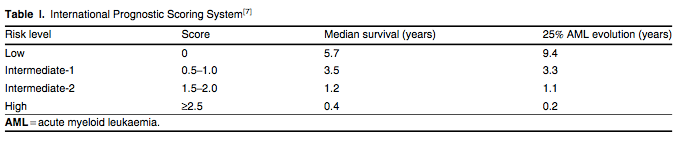
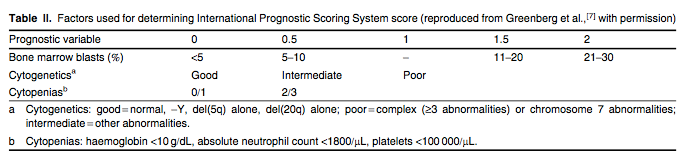
MDS is categorized according to the French American and British (FAB) criteria or by WHO classification. Due to the heterogeneity of the disease, several prognostic models have been designed to account for its complex biology.[5-7] The most widely accepted prognostic model is the International Prognostic Scoring System (IPSS). The IPSS divides de novo MDS patients into low, intermediate-1 (int-1), intermediate-2 (int-2) and high-risk categories based on the percentage of bone marrow blasts, cytogenetics and the number of cytopenias (tables I and II).[7] This scoring system predicts the risk of AML transformation and overall survival, as well as aiding treatment decisions. Important shortcomings of the IPSS are that it does not account for the following: (i) certain high-risk features of MDS such as therapy-related MDS and patients with cytogenetic aberrations involving chromosome 7 or patients with complex abnormalities; (ii) patients with chronic myelomonocytic leukaemia (CMML); (iii) previously treated patients; and (iv) prognostic implications of transfusion dependency.[8,9] Therefore, newer classification systems have been proposed such as the WHO Prognostic Scoring System and the MD Anderson model. These models can be applied to a broader range of patients and the latter model also improves prognostic prediction for all patients with MDS.[10,11]
正常狀態:
2. Principles of Therapy
Until recently, there were no approved drugs for the treatment of MDS. Supportive care measures (e.g. transfusion of blood products, haematopoietic growth factors and antimicrobials) remained the mainstay of therapy. Within the last 6 years, three agents have been approved by the US FDA for the treatment of MDS: the immunomodulating agent lenalidomide and the hypomethylating agents azacitidine and decitabine. These agents have dramatically changed both the course of treatment for MDS and patient outcomes. With that being said, allogeneic stem cell transplant remains the only potential cure and clinical trials should remain a viable option for all patients with MDS.
Factors that are important to consider when determining the therapeutic plan for patients with MDS include WHO or FAB classification, IPSS score, cytogenetic abnormalities, age, performance status and donor availability. Based on IPSS risk and bone marrow blasts, patients can be divided into lower-risk MDS (IPSS low or int-1, blasts <10%) and higher-risk MDS (IPSS int-2 or high, blasts ≥10%).[12] Generally, lower-risk patients are treated with low-intensity programmes striving for haematological improvement (HI) and high-intensity programmes are reserved for achieving remission in higher-risk patients. Table III outlines treatment options for MDS by IPSS risk category.

Immunosuppressive Therapy
A subgroup of patients with MDS will display similar characteristics to acquired aplastic anaemia. In this case, the presence of cytopenias without blasts may be caused by clonal T-cell-mediated suppression. Thus, these patients have a higher likelihood of responding to immunosuppressive therapy (IST) with antithymocyte globulin (ATG) with or without the addition of ciclosporin and/or corticosteroids.[1] Several studies have identified different characteristics that correlate with response to IST. In general, patients thought to have the best chance of response to IST include younger patients (≤60 years of age) with low or int-1 disease, and those with hypocellular bone marrows, shorter duration of RBC transfusion requirements, and those expressing D-related human leukaemic antigen 15 (HLADR15) or possibly paroxysmal nocturnal haemoglobinuria clone positivity.[46-49] Treatment with these agents has shown highly variable response rates between 16% and 50% with variable remission durations.[49-51] Due to the poor tolerability of ATG, especially among elderly patients, candidates for IST need to be carefully selected.
Imatinib and Lenalidomide
Cytogenetic analysis is important in determining therapy for patients with MDS. A small subgroup of patients with CMML have a PDGFRβ (platelet-derived growth factor receptor β) gene translocation involving chromosome band 5q33. Constitutive activation of this fusion gene yields a receptor tyrosine kinase that can be inhibited by imatinib. Data from case reports indicate that CMML patients with the PDGFRβ rearrangement may have a durable response to imatinib.[52,53]
Patients with an interstitial deletion of chromosome 5q [del(5q)] have shown high response rates with the immunomodulatory agent lenalidomide. Lenalidomide is a 4-amino-glutarimide analogue of thalidomide that is more potent and associated with an improved adverse effect profile. Like thalidomide, lenalidomide has similar antiangiogenic and immunomodulatory activity. Although thalidomide was the first immunomodulating agent used for the treatment of MDS and produced moderate response rates at escalating doses,[54] its use was strongly limited by drug toxicity; thus, lenalidomide became an attractive agent for the treatment of MDS.
Based on the results of two clinical trials (MDS-001 and MDS-003),[55,56] lenalidomide was FDA approved in 2005 for the treatment of lower-risk (IPSS low or int-1) MDS with del(5q), at an oral dose of 10 mg/day.
A phase I/II study (MDS-001) evaluated the safety and efficacy of lenalidomide among 43 MDS patients with transfusion-dependent or symptomatic anaemia, who either had no response to rhEpo or had elevated endogenous erythropoietin levels (>500 mU/mL). Patients with del(5q) were noted to have the strongest response rates to lenalidomide.[55] This observation led to a multicentre phase II trial (MDS-003). This trial evaluated the efficacy and safety of lenalidomide, administered at a daily dose of 10 mg continuously (amended schedule), in low or int-1 risk MDS patients with transfusion-dependent anaemia and del(5q) alone or in combination with other cytogenetic abnormalities. Among 148 patients, 67% achieved transfusion independence, regardless of the karyotype complexity. The median time to response was 4.6 weeks with a median duration of transfusion independence of 2.2 years. Additionally, 45% of evaluable patients (38/85) had a complete cytogenetic remission.[56]
Although patients without the presence of del(5q) can respond to lenalidomide, these response rates remain low. Another phase II study (MDS-002) evaluated the efficacy of lenalidomide in 214 low- or int-1 risk MDS patients with transfusion-dependent anaemia and without del(5q). Of the 214 patients, 26% achieved transfusion independence after a median of 4.8 weeks of therapy. The median duration of transfusion independence was 41 weeks.[57] Lenalidomide is an option for lower-risk MDS patients with anaemia not associated with del(5q) who remain transfusion dependent after supportive care or who do not qualify for other therapies.
Despite the convenience of an orally administered drug, lenalidomide is not without toxicity. The most common grade 3/4 adverse events were neutropenia and thrombocytopenia (55% and 44%, respectively, in the MDS-003 trial and 30% and 25%, respectively, in the MDS-002 trial).[56,57] Neutropenia and thrombocytopenia generally occur early in the treatment course and the majority of patients require dose reduction or interruption. In addition to myelosuppression, dose adjustment for renal impairment is required. Due to potential teratogenicity, lenalidomide is only available under a special restricted distribution programme. Under the RevAssist® programme, only prescribers and pharmacists registered with the programme can prescribe and dispense lenalidomide. In addition, lenalidomide must only be dispensed to patients who are registered and meet all the conditions of the programme.
Hypomethylating Agents
DNA methylation leads to the silencing of genes responsible for cell differentiation. In cancer, including MDS, hypermethylation suppresses normal regulator genes, allowing for tumour resistance and progression. Thus, re-expression of these silenced genes can be achieved by reversing the DNA hypermethylation. Azacitidine and decitabine inhibit DNA methyltransferase, resulting in hypomethylation of DNA and re-expression of tumour suppressor genes.[58]
Azacitidine
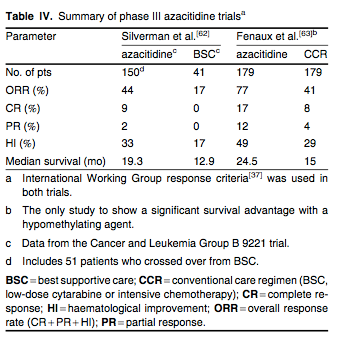
Azacitidine, a pyrimidine nucleoside analogue, was originally developed in the 1970s for use at high doses as a cytotoxic and leukaemic agent similar to cytarabine. Subsequently, phase II studies conducted by the Cancer and Leukemia Group B (CALGB) in MDS demonstrated tolerability and response rates of around 50% when the drug was given at lower doses for 7 days either as a continuous infusion or subcutaneously.[59,60] Based on these results, Silverman et al.[61] conducted a phase III randomized trial (CALGB 9221) that compared the safety and efficacy of subcutaneous azacitidine 75 mg/m2/day for 7 days of a 28-day cycle with best supportive care (BSC) in 191 patients with MDS. Treatment was continued for a minimum of four cycles before response criteria were assessed and the trial allowed for crossover to the azacitidine arm for patients with progressive disease on BSC. Nearly half of all evaluable patients had int-2 or high-risk MDS. Using non-IWG response criteria, responses occurred in 60% of azacitidine-treated patients (7% complete response [CR], 16% partial response [PR], 37% HI) compared with 5% improvement in the BSC arm (p < 0.0001). The median time to response was three cycles and the median duration of response was 15 months. Additionally, patients treated with azacitidine experienced a lower likelihood of transformation to AML or death than those on BSC (21 vs 13 months, respectively; p = 0.007), as well as an improved quality of life. Due to the crossover design of the study, there was no statistically significant improvement in overall survival with azacitidine compared with BSC. As part of the New Drug Application process, the FDA mandated the data in this trial along with the two other CALGB trials[59,60] be reanalysed using the IWG response criteria and WHO diagnostic classification. These data confirmed similar response rates with azacitidine in MDS patients[62] (summarized in table IV). Of note, reanalysis of the CALGB 9221 data showed that 17% of patients treated with BSC had HI compared with the 5% that was initially reported.[62]
Decitabine
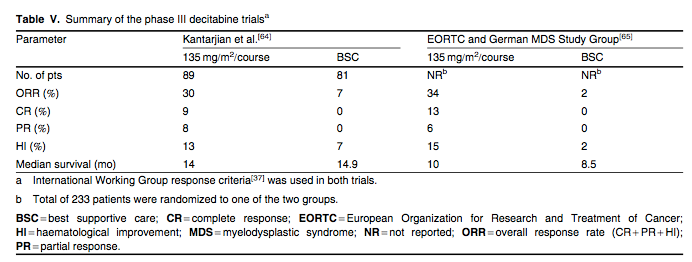
Decitabine was approved by the FDA in 2006 for the treatment of MDS on the basis of a phase III multicentre trial. This trial, summarized in table V, randomized 170 patients with MDS to either intravenous decitabine 15 mg/m2 over 3 hours every 8 hours for 3 consecutive days (135 mg/m2/course) every 6 weeks or BSC.[64] The majority of patients had int-2 or high-risk disease. The overall response rate for the decitabine arm was 30% (CR 9%, PR 8% and HI 13%) compared with 7% for the BSC arm (p < 0.001) by IWG[37] criteria. The median duration of response was 41 weeks with no difference in time to AML progression compared with BSC.[64] Similarly, the European Organization for Research and Treatment of Cancer and the German MDS Study Group conducted a large phase III, multicentre trial that randomized 233 elderly patients with higher-risk MDS to either intravenous decitabine 15 mg/m2 over 3 hours every 8 hours for 3 consecutive days (135 mg/m2/course) every 6 weeks or BSC.[65] This trial showed a 34% overall response rate with decitabine and an improvement in progression-free survival (0.55 vs 0.25 years; HR = 0.68; 95% CI 0.52, 0.88; p = 0.004) compared with the BSC group. However, there was no significant difference in time to AML progression or overall survival between the two groups.
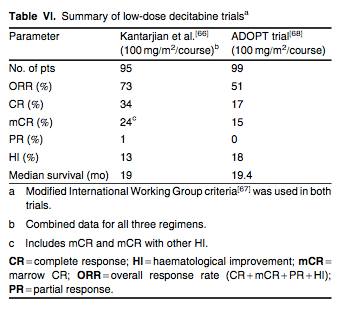
To evaluate the effect of decitabine on epigenetic modulation (hypomethylation), a phase II, single-centre trial randomized 95 patients to one of three low-dose decitabine treatment arms: intravenous 10 mg/m2 over 1 hour daily for 10 days, intravenous 20 mg/m2 over 1 hour daily for 5 days or subcutaneous 20 mg/m2 daily for 5 days (100 mg/m2/course) every 4 weeks.[66] Overall, 73% of patients demonstrated objective responses (includes CR, marrow CR, PR and HI) by modified IWG[67] criteria. The intravenous 20 mg/m2 over 1 hour daily for 5 days schedule produced the best clinical results, with a CR rate of 39%. The median number of cycles given was nine. Additionally, this low-dose, dose-intensive schedule of decitabine was associated with optimal hypomethylation.[66] The clinical efficacy of the 5-day low-dose decitabine regimen was confirmed in a phase II, multicentre, single-arm study (ADOPT [Alternative Dosing for OutPatient Treatment] trial).[68] In this trial, 99 patients were treated with intravenous decitabine 20 mg/m2 daily for 5 days every 4 weeks. Patients received a median of five cycles of treatment. The overall response rate by modified IWG[67] criteria was 51% (includes CR, marrow CR, PR and HI) with a median survival of 19.4 months.[68] Results of these two trials are summarized in table VI.
Investigational Agents
There are several investigational agents underway for the treatment of MDS. These agents belong to several different classes of drugs that target various biological and molecular mechanisms believed to play a role in the development of MDS (table VII). Numerous phase I and II studies with these agents as monotherapy as well as in a variety of combination regimens have been conducted or are currently ongoing. We highlight some of these promising agents in this section.
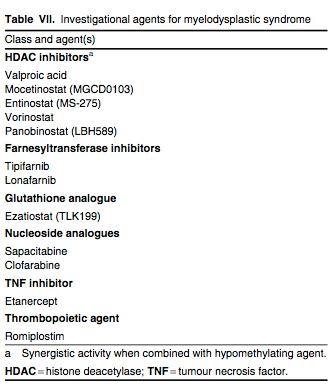
Histone Deacetylase Inhibitors
Maximizing epigenetic modulation plays a critical role in the treatment of MDS. In addition to gene silencing via DNA hypermethylation, gene silencing can occur through histone deacetylation, which is regulated by histone deacetylase (HDAC) enzymes.[58] Several HDAC inhibitors are under clinical investigation for the treatment of MDS (table VII). Valproic acid, a commercially available antiepileptic drug, has been the most widely studied HDAC inhibitor in MDS. As monotherapy, valproic acid generates modest to moderate response rates of 16–44%, with the higher end of response rates associated with lower-risk disease.[76] However, when combined with hypomethylating agents, the combination shows synergistic effects in vitro.[58] A phase I/II study evaluated the combination of azacitidine with valproic acid and tretinoin (all-trans retinoic acid) in 53 patients with high-risk MDS and AML. The overall response rate was 42%, with a median remission duration of 26 weeks. Of note, the median time to response was after only one course of therapy. The dose-limiting toxicity was reversible neurotoxicity.[77]
In another phase I/II study, escalating doses of valproic acid were combined with decitabine in 53 evaluable patients with AML and MDS. An overall response rate of 22% (CR 19% and CR with incomplete platelet recovery [CRp] 3%) was reported. Response rates of 50% (CR 40% and CRp 10%) occurred in the elderly population (five of ten patients).[78] Randomized studies addressing synergy with the addition of HDAC inhibitors to the hypomethylating agents are ongoing. More potent HDAC inhibitors such as vorinostat, entinostat (MS-275), panobinostat (LBH589) and mocetinostat (MGCD0103) are being studied both as monotherapy and in combination regimens.[58]
Clofarabine
Clofarabine is a purine nucleoside analogue. Its activity and safety was evaluated in two phase II studies in MDS patients.[79] Thirty-six patients were treated with intravenous clofarabine and randomized to receive 15 or 30 mg/m2/day for 5 days every 4–6 weeks. The 15 mg/m2 intravenous regimen produced higher response rates than the 30 mg/m2 regimen, with responses observed in 50% (CR 35%, CRp 15%) of patients. In a second study, 24 patients were treated with oral clofarabine and received a starting dose of 40 or 30 mg/m2/day for 5 days every 4–6 weeks. The oral formulation produced response rates in 50% of patients (CR 29%, CRp 8%, HI 13%). In total, six patients died, all of whom received intravenous clofarabine. Death was most commonly related to infectious complications. Additionally, seven patients developed acute renal failure.[79] Since lower doses of clofarabine demonstrated activity in MDS, additional dose-finding studies are in progress to determine a safe and effective dose for the treatment of higher-risk MDS.[80-83]
Farnesyltransferase Inhibitors
Farnesyltransferase is one of many enzymes responsible for the regulation of cancer cell growth and differentiation by activating proteins such as Ras. Inhibiting this enzyme prevents the activation of Ras and, ultimately, cell growth.[58] Tipifarnib and lonafarnib are the two main farnesyltransferase inhibitors (FTIs) under clinical investigation for use in MDS patients. Tipifarnib has been studied in small phase I and II trials at varying doses and schedules in MDS patients with response rates of 11–30%.[84,85] In a multicentre phase II trial of 82 patients with intermediate- to high-risk MDS, tipifarnib was administered orally at 300 mg twice daily for 21 days of a 28-day cycle. An overall response rate of 32% was reported, with 12 patients (15%) achieving CR and 14 patients (17%) with HI. CR occurred after a median of 4 weeks and lasted for a median of 12.5 months, while HI was achieved for 18 weeks.[86] The most common grade 3/4 toxicities with tipifarnib were myelosuppression and neurological toxicity. Phase I and II trials of lonafarnib in MDS have shown similar response rates of 20–30%.[87] Unlike tipifarnib, lonafarnib was associated with significant grade 3/4 gastrointestinal toxicity.[87]
Romiplostim
Romiplostim, a thrombopoiesis-stimulating peptibody, was recently FDA approved for the treatment of chronic immune (idiopathic) thrombocytopenic purpura. Its mechanism of action has made this agent of interest in the treatment of patients with lower-risk MDS and thrombocytopenia. Kantarjian et al.[88] treated 44 patients with lower-risk MDS and platelet counts ≤50 × 109/L with romiplostim 300–1500 μg subcutaneously weekly for three cycles. Platelet counts increased to >100 × 109/L for ≥8 weeks in 13 of 18 responders. Six patients had a transient increase in blasts, which resolved within 7 weeks after discontinuation of the drug. All six of these patients were treated with a dose ≥1000 μg. Additionally, in a randomized, multicentre, phase II study by Kantarjian et al.,[89] romiplostim was shown to reduce clinically significant thrombocytopenic events and platelet transfusions, and improve platelet nadirs in low- and intermediate-risk MDS patients receiving azacitidine. Similarly, preliminary data from ongoing studies using romiplostim as monotherapy as well as in combination with other agents such as decitabine and lenalidomide for patients with MDS are emerging.[90-92]
Conclusions
Supportive care measures, consisting of blood and platelet transfusions, haematopoietic growth factors and antimicrobials, remained standard of care for the treatment of MDS patients until the recent emergence of three FDA-approved agents: lenalidomide, azacitidine and decitabine. In addition, the further development of classification systems and prognostic models has contributed significantly to advancements in how MDS is treated. Clinicians are now able to provide patient-tailored therapy for specific MDS subgroups. For example, the use of lenalidomide for the treatment of del(5q) lower-risk patients has proven strong response rates and is now considered first-line therapy for this distinctive population of patients. The hypomethylating agents decitabine and azacitidine have significantly improved outcomes for patients with higher-risk MDS. Furthermore, one of the most significant advances in MDS came with the recent finding that azacitidine was associated with an improved overall survival when compared with conventional care regimens for patients with higher-risk MDS. This is the first agent for MDS to show an improvement in overall survival. This progress highlights the need for future randomized phase III clinical trials in this complex disease. Clinical trials addressing combination therapies with multiple investigational agents as well as novel combination regimens are ongoing.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 