A new generation of genomic technologies permits the increased collection of data on large study populations.1,2 New methods in informatics facilitate the integration of diverse types of information with genomic data in disease research. As a result, researchers are learning more about the genetic bases of disease and response to drugs.3-6 Genetic tests, including many that are offered directly to the consumer, are growing in number and clinical relevance. Genomic knowledge and technologies are also being adopted in areas distant from human health. Here, I describe evolving policies pertinent to genetic and genomic research, the integration of genetics into clinical care, and the broader issues raised by genetic technologies and information.
CONTROVERSIES IN GENOMIC AND GENETIC RESEARCH
Although genetic and genomic research do not raise wholly new ethical issues in the context of general biomedical research, they cast these issues in a fresh light. First, research using collections of biologic specimens, genomic data, and information from medical records has amplified the long-standing yet unresolved issue about consent for future research that is unanticipated at the time of specimen collection. Second, the push for broad access to research data sets has raised privacy concerns. Third, as researchers seek to share data with colleagues, the issue of whether and how to share research results with study participants remains vexing, particularly in the absence of explicit prior consent from participants.
Consent and Confidentiality
Consent documents provide a means of communicating the risks of a study (including informational risks) to participants. Narrow consent documents describe the benefits and risks of a specific study, whereas a broad consent may ask participants to agree to any number of future studies that will use their samples or data. Research participants may vary in their views and preferences about consent and in whether they wish to undergo a reconsenting process for every specific study that may be conducted with their specimens or information. They may also vary in their views about being notified of the results and implications of such studies. Broad consent is often obtained for future research on stored specimens and medical records because such research is considered to be of low risk to participants. Having the option of giving broad consent for future studies that use a stored sample or data set provides participants with a measure of control over their personal information and specimens. As vividly described in the book The Immortal Life of Henrietta Lacks, the process of obtaining consent by asking permission to use samples or data is a potent expression of respect.7
The emergence of new ideas and new technologies for analyzing samples and data has raised important and controversial issues about whether and how to recontact participants to seek additional consent. Successful recontact requires a level of traceability among participants, which may increase the risk of breach of privacy, be overly intrusive, and be expensive, time-consuming, and cumbersome for researchers as participants move around or change names.8 However, the Internet and social networking have made it much easier to maintain or reestablish contact with participants.
Broad consents do not capture informed consent about all future uses of a participant's sample or data. However, they do provide researchers with more flexibility. There is a middle ground between strictly narrow and overly broad consent; researchers can provide limitations on investigators or institutions that would have access to the sample or data and the types of studies that would be considered.9 Tiered consent allows participants to specify preferences for the future use of their samples or data. Participants can choose to opt out of all future studies, be contacted before any future use, allow unlimited use of their de-identified sample, or even specify the types of studies in which they would want or would not want their samples to be used.10,11
The regulations that govern research on human subjects have their roots in the Tuskegee revelations of the early 1970s and were designed primarily to protect against physical and psychological risks in research. They touch on informational risks only tangentially.12 However, researchers do need to describe to potential participants, as well as to grant-giving bodies, how the confidentiality of each participant is to be maintained.
Research that was not contemplated in the original consent and that uses de-identified data and specimens is not considered human-subjects research and is not subject to the regulations protecting research participants.13 Thus, the elimination of identifiers can remove the need for consent and the ability to provide information to the subject. Indeed, this interpretation of the regulations might even be seen as providing an incentive to researchers to remove identifiers. However, true de-identification of biologic specimens or genomic data is not always possible, because a small number of genetic variants can uniquely identify a participant.8,14 Even aggregate data (e.g., pooled data from several hundred subjects) may not be safe; a forensic technique of DNA analysis allows for the determination of whether a subject with a specific genetic profile has contributed to aggregate genomic data.15 The identifiability of genomic data is especially important, given that privacy is a major concern of potential research participants.16
To protect research participants, researchers collecting sensitive and identifying data can request a certificate of confidentiality from the Department of Health and Human Services (HHS) (Table 1).
TABLE 1Quick Guide to Certificates of Confidentiality.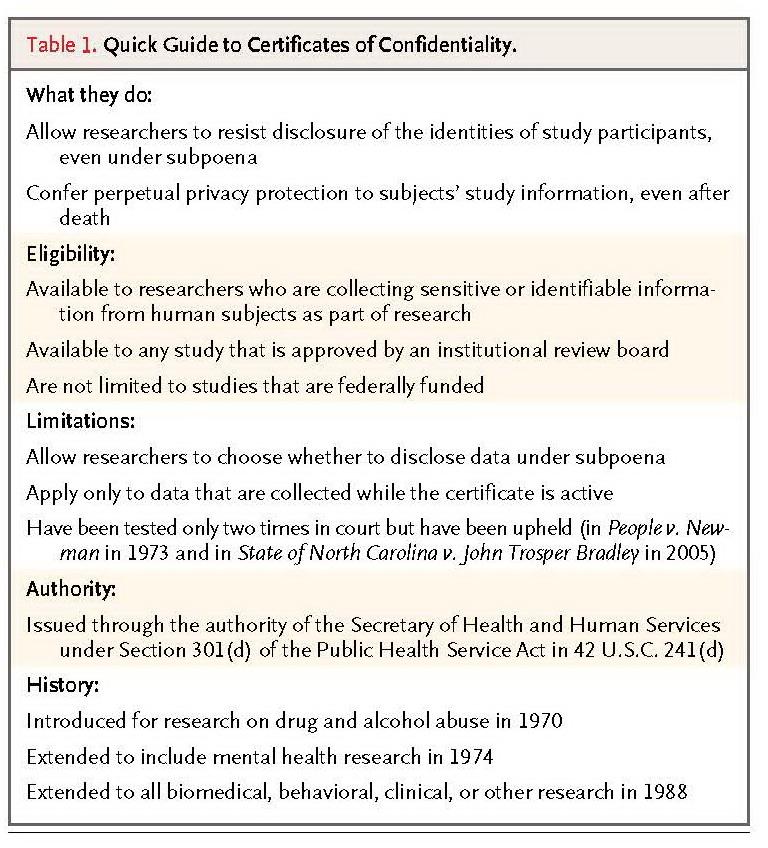
These certificates authorize researchers to avoid compelled disclosure “in any Federal, State or local civil, criminal, administrative, legislative, or other proceedings.”17 There are two important limitations. First, certificates of confidentiality do not require researchers to refuse to disclose identifying information; they only convey the legal right to do so. It is possible to imagine a circumstance in which law-enforcement officials might argue that disclosure of research information could help solve a string of violent crimes. The research institution and community might bring substantial pressure to bear on the researcher, who might feel obligated to disclose the requested information. Such cases are only imaginary now, but as large biobanks proliferate, they are likely to occur, and the disclosure to law enforcement could lead to distrust in the ability of the research enterprise to protect privacy. Second, certificates are not mandatory. Only a small percentage of researchers seek certificates of confidentiality. One survey revealed that only 114 such applications were submitted from more than 27,000 studies that were funded by the National Institutes of Health (NIH). Fifty-four percent of the researchers who did submit applications cited genetic information as the reason.18However, there are models of mandatory protection. The National Institute of Justice, the research division of the Department of Justice, requires all its investigators to have a privacy certificate that compels them to keep identifiable data confidential.19 If both data sharing and protecting the privacy of participants are to be preserved, stronger systematic safeguards may be needed.
HHS has recently proposed major reforms to the regulations governing human-subjects research. The proposed reforms would touch on many of the issues described here, including consent for research use of biospecimens and the requirements for consent and review by an institutional review board for research with biospecimens.20,21
Return of Research Results
Whether, when, and how to return results of genetics research to participants have long been the subject of ethical and policy debate.22,23 These issues have only grown more complex as it has become clear that some research participants want to have access to research results or at least have the option of choosing whether to receive results.24-26 Survey respondents who were asked about their willingness to participate in a proposed cohort study said that receiving the individual results of research was the most important inducement.25 Some observers have argued that the reporting of results, especially clinically actionable results, is a medical and ethical obligation.22
There are many hurdles to delivering such information. In the United States, test results can be reported only by laboratories that are certified according to the provisions of the Clinical Laboratory Improvement Amendments (CLIA).27 In addition, data from research are often preliminary and need to be validated. However, even some of the most well-validated variants that are associated with disease, especially those that have been uncovered by new genomic approaches, have a small effect on disease risk, and such results can be difficult to explain and to understand. The reporting of results that have not been validated or those with questionable clinical benefit would require many disclaimers.
INTEGRATION OF GENETICS INTO MEDICINE
The successful integration of genetic testing into medicine requires an educated health care workforce, protections against inappropriate disclosure and discriminatory use of genetic information, and an oversight system that ensures the accuracy and reliability of genetic tests, particularly tests that provide results pertinent to important medical decisions. The dramatic increase in the number, complexity, availability, and medical relevance of genetic tests has created many regulatory challenges, as well as opportunities for change.
Regulation of Genetic Tests
Today's system of regulating medical-testing laboratories was put in place more than 20 years ago, when the Human Genome Project was just a wild notion and sequencing a patient's genome to diagnose a disease was virtually unimaginable. In enacting CLIA in 1988, Congress sought to ensure accurate, reliable, and timely testing. CLIA oversees clinical laboratories and, by extension, their performance of laboratory-developed tests. Although the Food and Drug Administration (FDA) has the legal authority to regulate laboratory-developed tests, including genetic tests, they have opted to regulate only test kits and some components of such tests. Thus the overwhelming majority of genetic tests are not currently subject to FDA scrutiny.
In 2008, the Secretary's Advisory Committee on Genetics, Health, and Society issued recommendations for the reform of genetic-testing oversight, including that CLIA require laboratories to participate in proficiency testing for laboratory-developed tests, that the FDA begin risk-based evaluation of such tests, and that HHS develop a mandatory test registry.28 Recently, the FDA has announced its intention to begin risk-based oversight of laboratory-developed tests, and the NIH is developing a genetic-testing registry to provide a centralized source of information on the more than 1600 genetic tests available to patients and other consumers.29-31 These steps reflect a shared emphasis by the NIH and the FDA on “personalized medicine and the scientific and regulatory structure needed to support its growth.”32
Pharmacogenetics
One of the most promising areas of genomic medicine is the ability to match an individual's genetic profile to the likely effect of particular drugs (see Glossary). Genetic makeup can predict the occurrence of toxic effects, such as the hypersensitivity reaction that occurs in carriers of the HLA-B*5701 allele who receive abacavir for the treatment of human immunodeficiency virus infection.33,34 The presence of various mutations in tumors can also predict the efficacy of certain drugs, such as cetuximab and panitumumab for colorectal cancer, since tumors that contain certain somatic mutations in KRAS do not respond to these drugs.35-38 In addition, the findings of genomic research can be used to identify promising drug targets. Sequencing of genes encoding kinases led to the discovery of a BRAF mutation in melanoma, which in turn led to the development of PLX4032, a drug that was designed to inhibit the mutant BRAF protein and that brought about tumor regression in a majority of patients in a phase 1 clinical trial.39
According to the FDA, 77 approved drugs contain pharmacogenomic information in their labeling,40but in many cases, the labels do not provide action-oriented information for physicians and patients. For example, the label for the antidepressant protriptyline generally states that the drug is metabolized by CYP2D6 and that some patients may have poor metabolization.41 This information is probably of limited use to most physicians and patients. In contrast, the most recent label for warfarin includes a table showing the appropriate starting dose on the basis of the patient's CYP2C9and VKORC1 genotype combination, which should be more useful to physicians.42 Table 2.
TABLE 2Examples of Drugs with Genetic Information in Their Labels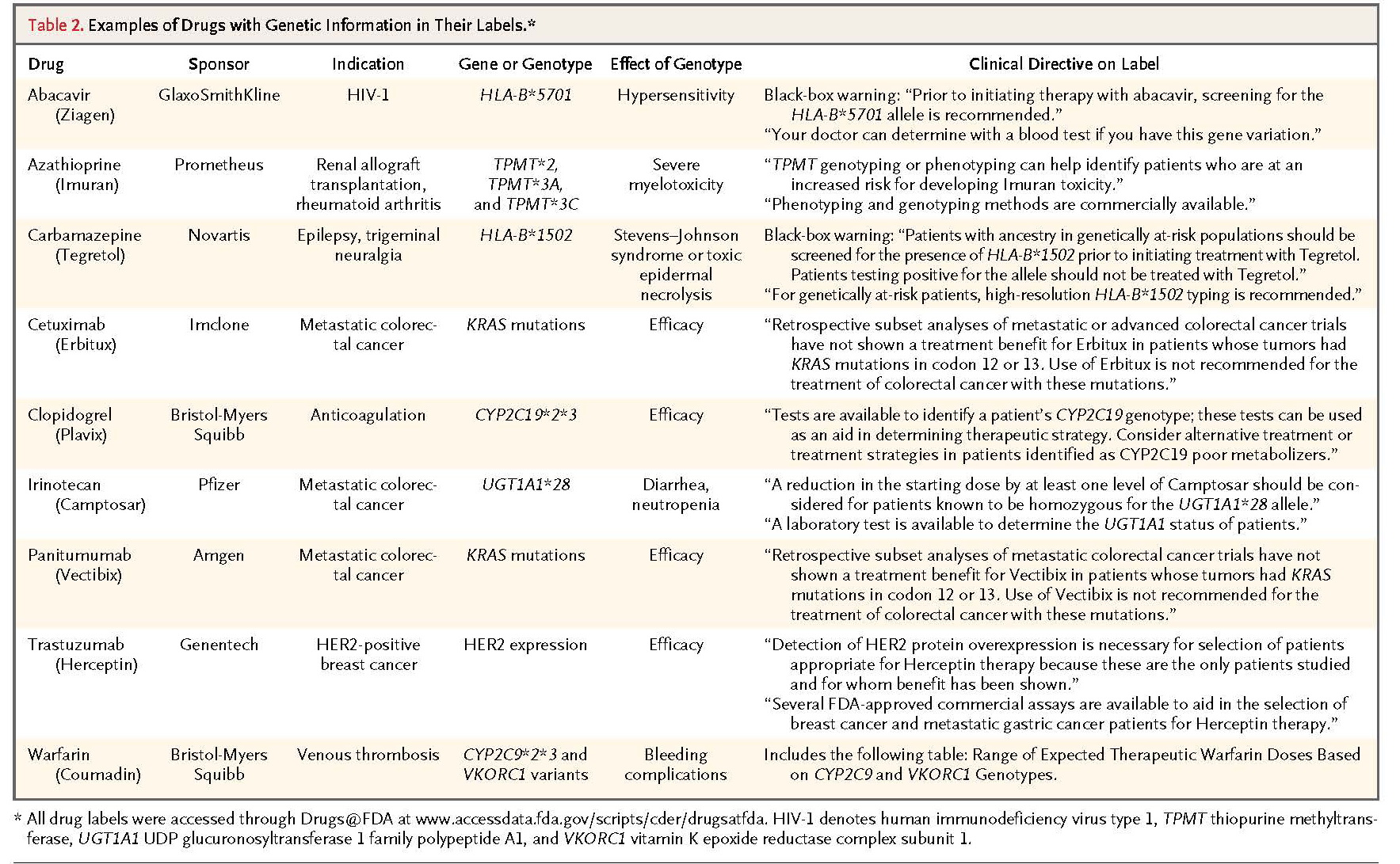
lists some examples of drugs with genetic information in their labeling.42-50 The inclusion of clear and comprehensive drug labeling that informs about genetic tests relevant to the safety or efficacy of use is critical to enhancing patient care.
ELECTRONIC MEDICAL RECORDS
In a world of electronic medical records (EMRs), there is a dual challenge of usefully incorporating genetic information while also protecting patient privacy. Consumers, health care providers, insurers, and regulators face a difficult balancing act to protect the privacy of genetic and other health information while also ensuring its availability and use for medical decision making.
The use of EMRs is becoming well established in some health care systems, such as those of Kaiser Permanente and the Geisinger Health System. However, according to the results of a survey of health care professionals who use 10 different EMR systems, the integration of genetic information is lagging behind. Only 4% of the respondents reported that their EMR system provided any decision support on the basis of the results of genetic tests, and the vast majority reported that their EMR supplier did not provide the type of support they needed for the interpretation of genetic information.51
EMRs could provide a platform for the integration of genetic information into clinical practice by guiding clinicians about when to order a genetic test, how to document and interpret the results, how to apply the information for treatment decisions and prevention screening, and when to refer patients for genetic counseling.51,52 Such automated guidance may be vital for both health care workers and their patients. In addition, EMRs may facilitate research with large cohorts, a factor that is especially valuable for prospective studies of genetic and environmental effects on health in which the linking of phenotype to genotype is essential.
The ease with which information can be shared across electronic databases increases the risk of unauthorized use and access to the information.52 This risk is relevant not only to patients but also to their family members, who will share many genetic loci with the patients and with one another. Unlike other clinical information, genetic information is largely immutable. If whole-genome sequences are eventually included in medical records, they can be reanalyzed as more disease-risk loci are identified, which may lead to more incidental findings in clinical care.
The privacy rule outlined in the Health Insurance Portability and Accountability Act (HIPAA) sets standards for how “protected health information” (i.e., essentially any individually identifiable health information) should be controlled.53 HIPAA's privacy rule applies only to “covered entities,” which are defined as health care providers who electronically transmit any health information in connection with transactions for which HHS has adopted standards, health plans, and health care clearinghouses.54 Under this definition, many facilities that perform direct-to-consumer genetic testing and analysis are exempt.55 In effect, the regulation does not address the type of information that is protected but, rather, who holds it. In a rapidly changing medical marketplace, there are many open questions about how to best protect the privacy of patients and consumers while also promoting research and the quality of clinical care.
As originally written, the HIPAA privacy rule did not explicitly provide privacy protections for genetic information. Part of the Genetic Information Nondiscrimination Act of 2008 (GINA) requires that HHS amend the HIPAA privacy rules to correct this oversight (Table 3
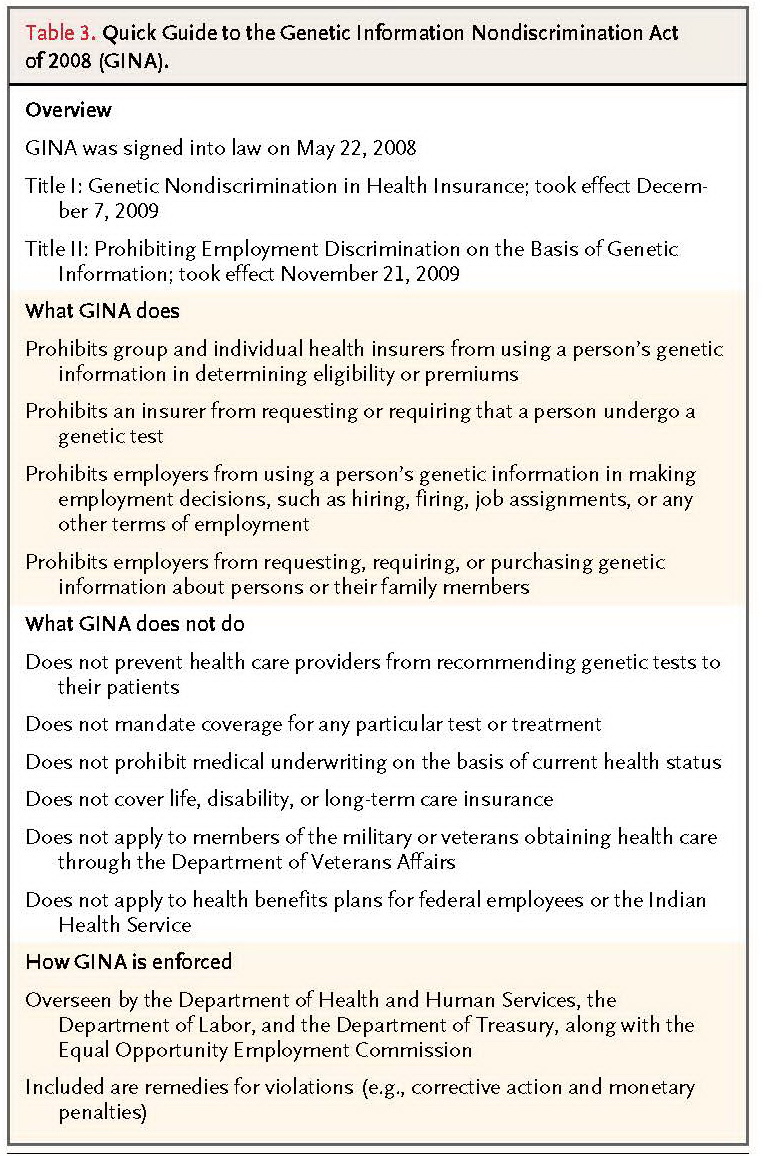
TABLE 3Quick Guide to the Genetic Information Nondiscrimination Act of 2008 (GINA).
). In October 2009, HHS issued a proposed rule to, among other things, revise the definition of “health information” to explicitly include genetic information and add GINA's statutory definition of “genetic information” to the privacy rule.56As of this writing, the final rule has not been issued.
GENETIC DISCRIMINATION
GINA was designed to address concerns that information from genetic testing could lead to new forms of discrimination. It was hailed by its supporters as “the first civil-rights bill of the new century of life sciences.”57GINA's prohibitions on genetic discrimination in health insurance took effect on December 7, 2009. Under these rules, insurers in the group and individual health insurance market cannot use genetic information to increase premiums, deny enrollment, or impose exclusions for preexisting conditions. Insurers cannot request, require, or buy genetic information for underwriting purposes and are generally prohibited from asking individuals or family members to undergo a genetic test.
HIPAA was the first piece of federal legislation to protect against genetic discrimination in employer-sponsored group health plans. Specifically, genetic information cannot be considered a preexisting condition and thus cannot be used as a basis for the denial of coverage.58 GINA extends this mandate by prohibiting the use of genetic information for underwriting purposes in group and individual health insurance. GINA protects against the use of test information and family medical history, but it does not limit the use of any information related to the manifestation of a disease or condition with a genetic component. Thus, a person with a family history of kidney disease who undergoes genetic testing and is found to have a dominant gene mutation would be protected under GINA. However, information resulting from imaging studies that may identify renal cysts would not be protected under GINA.
The 2010 Patient Protection and Affordable Care Act (ACA) outlaws discrimination by health insurers on the basis of signs and symptoms of genetic disease. Group or individual health insurers cannot establish rules for eligibility on the basis of health status, medical condition, claims experience, receipt of health care, medical history, genetic information, evidence of insurability, or disability.59 Beginning in 2014, the ACA will prohibit variations in premiums according to health status and genetic information.
GINA stipulates that it is unlawful for employers to make employment-related decisions on the basis of genetic information. In addition, it is illegal for an employer to request, require, or purchase employees' genetic information except under narrowly defined circumstances. Because of GINA, employees in the United States have a private right of action for reinstatement, back pay, compensatory and punitive damages, attorney's fees, and other relief.60
GINA's provisions do not apply to life insurance, disability insurance, or long-term care insurance. GINA also does not apply to members of the military, veterans obtaining health care through the Department of Veterans Affairs, health benefits plans for federal employees, and the Indian Health Service. The effects of some of these limitations have been largely mitigated by the ACA and by policies for fair use of genetic information by the military and the Department of Veterans Affairs.61GINA also does not apply to educational or athletic programs. For example, the National Collegiate Athletic Association began mandatory testing for sickle cell trait among students participating in Division I athletics.62
Law Enforcement
The collection and use of DNA by law enforcement and the courts for identification and prosecution of criminals have been standard practice since the mid-1980s. The Combined DNA Index System (CODIS), a fully integrated law-enforcement system of DNA records, contains more than 9.1 million DNA profiles from convicted offenders and more than 346,000 DNA profiles obtained from crime scenes.63 CODIS is credited with aiding in more than 127,000 investigations. In addition to aiding in convictions, DNA testing has been used to exonerate 273 wrongly convicted persons in the United States.64 In January 2011, a Texas man was found to have been innocent of a crime that had kept him behind bars for 30 years.65
Although the use of DNA evidence in law enforcement has tremendous potential to promote justice, it also raises profound legal, ethical, and social concerns. Among these are concerns about privacy and the disparate effect on historically vulnerable populations that may deepen the racial inequalities in the criminal justice system.66 Only persons who come into contact with law enforcement are entered into the database. Racial and ethnic minorities are disproportionately represented in these databases. Such persons will be more likely than whites to be identified by DNA profiles, since only DNA profiles that are included in databases can be matched to DNA evidence from a crime scene. To overcome this problem and further improve law enforcement, some observers have advocated for the creation of a uniform national DNA database.
Pros and Cons of Gene Patents
The court's ruling in Diamond v. Chakrabarty 67 that an engineered microorganism was patentable subject matter paved the way for a 30-year explosion in intellectual-property cases in biology that was fueled by the expansion of knowledge and methods in biotechnology, particularly in sequencing and genetic testing. By 2010, there were patents on an estimated 20% of the human genome, including patents on both genetic sequence and methods for its analysis.68 The United States has been the hub of gene patenting, with more than twice as many patent applications as in Europe.69The effects of gene patents on innovation, investment, and access to patients have been hotly debated.68 The plummeting costs of DNA sequencing have raised questions about whether the clinical or commercial use of whole-genome sequencing would infringe on gene patents and whether the patent holders would enforce their intellectual-property rights and, if so, under what terms.70These issues are at the heart of a current lawsuit that could have far-reaching implications for research, the biotechnology industry, and medicine.
In May 2009, the Association for Molecular Pathology and others filed suit against Myriad Genetics, arguing that the patents on the sequence of BRCA1 and BRCA2 were unconstitutional. This case is only the most recent twist in the tale of two genes that are perhaps the best known of all the genes in the human genome. BRCA1 and BRCA2 came to public attention and became the source of conflict during the race for their discovery and have remained at the heart of legal, political, and public affairs debates around the world ever since. For example, although Myriad Genetics has exclusive rights in the United States to analyzing these genes in the context of determining genetic risk, Canada refused to use the testing services of Myriad's Canadian licensee, which resulted in very public and acrimonious debate.71
In March 2010, District Court Judge Robert Sweet issued a surprising ruling in this case. He held that the composition and method claims of Myriad's seven patents pertaining to BRCA1 and BRCA2were invalid. He stated that the isolated DNA is not “markedly different” from a product of nature and that the claimed processes do not specify transformative actions beyond merely analyzing or comparing.72 Myriad Genetics appealed the decision. In October 2010, in an unexpected twist, the Department of Justice filed an amicus brief arguing that complementary DNA and other engineered DNA molecules are man-made and thus are patentable but that isolated DNA that is simply separated from the rest of the genome and its cellular environment is not patentable. The brief read, “The BRCA genes, their deleterious alleles, and their relationship to breast cancer are the products of evolution, not human invention.”73
If the courts were to agree with the Department of Justice's position, patents on manipulated nucleic acid sequences that may prove useful as therapeutics — such as sequences of small interfering RNA or DNA sequences used in the context of gene therapy — would remain eligible for patent protection and thus continue to be attractive to investors. Such a decision would also permit whole-genome sequencing or variant detection of native genomic DNA without the danger of infringing on gene patents or requiring royalty payments. On July 29, 2011, the U.S. Court of Appeals for the Federal Circuit concluded that isolated DNAs are eligible for patents. The plaintiffs may appeal, so the final decision may not be known for some time.
CONCLUSIONS
The field of genomics is evolving at a dizzying pace. Researchers are producing genomewide data sets on ever-expanding study populations. Broad access to these data, stored samples, and EMRs are accelerating our understanding of the role of genes, environment, and behavior in health and disease. Translational research is converting new knowledge into diagnostics, targets for drug development, and new insights about how to prevent and treat disease. The challenge is to ensure that innovation in research and medicine is equaled by innovative policies that foster science and discovery while protecting and respecting research participants and patients.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 