BACKGROUND
Intravenous sodium bicarbonate and oral acetylcysteine are widely used to prevent acute kidney injury and associated adverse outcomes after angiography without definitive evidence of their efficacy.
METHODS
Using a 2-by-2 factorial design, we randomly assigned 5177 patients at high risk for renal complications who were scheduled for angiography to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride and 5 days of oral acetylcysteine or oral placebo; of these patients, 4993 were included in the modified intention-to-treat analysis. The primary end point was a composite of death, the need for dialysis, or a persistent increase of at least 50% from baseline in the serum creatinine level at 90 days. Contrast-associated acute kidney injury was a secondary end point.
RESULTS
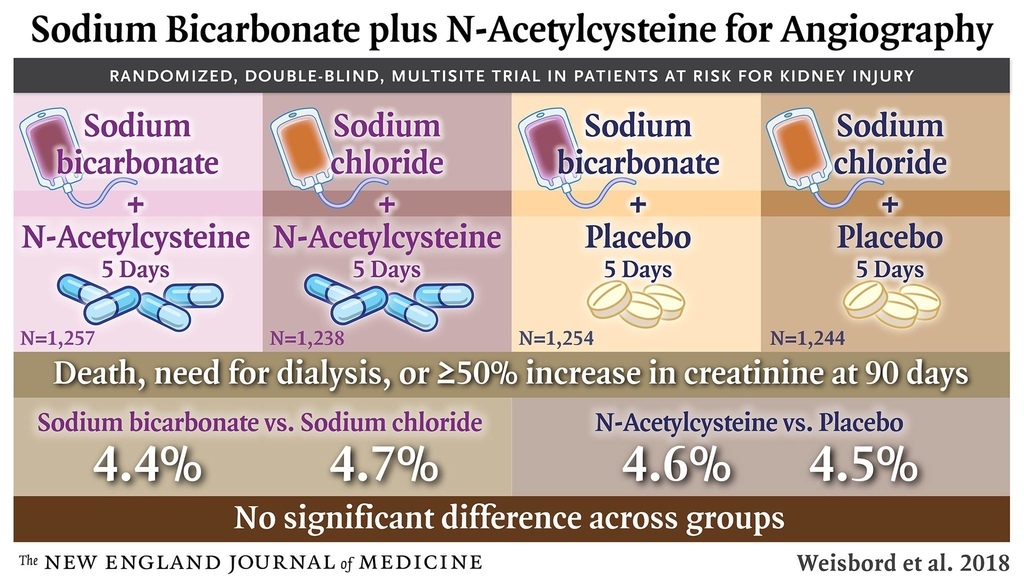
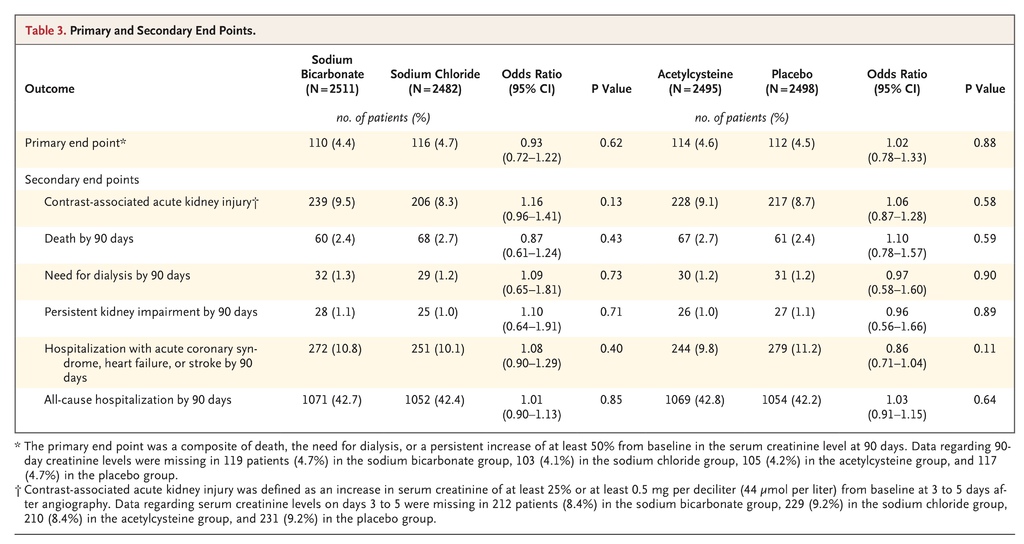
The sponsor stopped the trial after a prespecified interim analysis. There was no interaction between sodium bicarbonate and acetylcysteine with respect to the primary end point (P=0.33). The primary end point occurred in 110 of 2511 patients (4.4%) in the sodium bicarbonate group as compared with 116 of 2482 (4.7%) in the sodium chloride group (odds ratio, 0.93; 95% confidence interval [CI], 0.72 to 1.22; P=0.62) and in 114 of 2495 patients (4.6%) in the acetylcysteine group as compared with 112 of 2498 (4.5%) in the placebo group (odds ratio, 1.02; 95% CI, 0.78 to 1.33; P=0.88). There were no significant between-group differences in the rates of contrast-associated acute kidney injury.
CONCLUSIONS
Among patients at high risk for renal complications who were undergoing angiography, there was no benefit of intravenous sodium bicarbonate over intravenous sodium chloride or of oral acetylcysteine over placebo for the prevention of death, need for dialysis, or persistent decline in kidney function at 90 days or for the prevention of contrast-associated acute kidney injury. (Funded by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia; PRESERVE ClinicalTrials.gov number, NCT01467466.)
Acute kidney injury associated with the administration of contrast material during angiography can result in death, accelerated progression of underlying chronic kidney disease, and the need for dialysis, along with substantial increases in health care costs.1-3 The periprocedural administration of intravenous isotonic sodium chloride has been the standard intervention to prevent this complication.4 On the basis of hypotheses that urinary alkalinization and scavenging of reactive oxygen species mitigate renal tubular epithelial-cell injury from the use of iodinated contrast material, multiple studies have compared intravenous sodium bicarbonate with intravenous sodium chloride and have evaluated treatment with acetylcysteine for the prevention of contrast-associated acute kidney injury, with inconsistent results.5-11 Consequently, equipoise exists regarding these interventions, despite their widespread use in clinical practice. We designed the Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial to compare intravenous sodium bicarbonate with intravenous sodium chloride and oral acetylcysteine with oral placebo for the prevention of major adverse outcomes and acute kidney injury in a large population of high-risk patients undergoing coronary or noncoronary angiography.
Methods
TRIAL DESIGN AND OVERSIGHT
The trial was a double-blind, placebo and comparator-drug–controlled, randomized trial sponsored by the U.S. Department of Veterans Affairs Cooperative Studies Program and the George Institute for Global Health. From February 2013 through March 2017, patients were enrolled at 53 medical centers in the United States (35 Veterans Affairs sites), Australia (13 sites), Malaysia (3 sites), and New Zealand (2 sites). The design of the trial has been described previously.12 The trial protocol and statistical analysis plan are available with the full text of this article at NEJM.org. The trial was funded by the Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia.
The trial was approved by the Veterans Affairs central institutional review board and by relevant ethics committees and regulatory authorities at sites not overseen by the Department of Veterans Affairs. An independent data and safety monitoring committee met twice a year for the duration of the trial. Trial authors at the data coordinating center had access to the final results and vouch for the accuracy and completeness of the data and analyses. All the authors vouch for the adherence of the trial to the protocol. The first author drafted the manuscript, which was reviewed and approved by all the authors, who made the decision to submit the manuscript for publication. The Veterans Affairs Cooperative Studies Program reviewed the manuscript but did not influence the interpretation of the trial results or the decision to submit the manuscript for publication. Baxter Healthcare provided matched, prepackaged bags of sodium bicarbonate and sodium chloride at cost but was not involved in the design or conduct of the trial, data analysis, manuscript preparation, or manuscript review.
TRIAL POPULATION
Patients who were scheduled to undergo coronary or noncoronary angiography and who had an estimated glomerular filtration rate (eGFR) of 15 to 44.9 ml per minute per 1.73 m2 of body-surface area or 45 to 59.9 ml per minute per 1.73 m2 among those with diabetes mellitus were eligible to participate. The eGFR that was used to determine eligibility was calculated with the use of the four-variable Modification of Diet in Renal Disease Study equation on the basis of creatinine levels obtained locally within 30 days before angiography.13 We excluded patients who were undergoing emergency angiography and those with unstable baseline levels of blood creatinine (which was defined as an increase or decrease of ≥25% within 3 days before angiography). A complete list of inclusion and exclusion criteria is provided in Table S1 in the Supplementary Appendix, available at NEJM.org. All the patients provided written informed consent.
RANDOMIZATION, INTERVENTIONS, AND KIDNEY-FUNCTION MEASURES
Using a two-by-two factorial design, we randomly assigned eligible patients to receive intravenous 1.26% sodium bicarbonate (150 mmol per liter) or intravenous 0.9% sodium chloride (154 mmol per liter) in matched 1-liter bags (Baxter Healthcare), and oral acetylcysteine capsules (prepared using bulk powder from Moehs Catalana) or matched placebo capsules. Randomization was performed by means of a centralized, computer-generated permuted-block plan and was stratified according to trial site. The patients and trial investigators were unaware of trial-group assignments.
The administration of trial intravenous fluids was based on protocol-specified ranges: 1 to 3 ml per kilogram of body weight per hour during a period of 1 to 12 hours for a total volume of 3 to 12 ml per kilogram before angiography, 1 to 1.5 ml per kilogram per hour during angiography, and 1 to 3 ml per kilogram per hour during a period of 2 to 12 hours for a total volume of 6 to 12 ml per kilogram after angiography. Within these specific parameters, the providers at trial sites determined the timing of initiation, duration, and rate of fluid administration. In patients with a body-mass index (the weight in kilograms divided by the square of the height in meters) of more than 30, we capped fluid-administration rates on the basis of a weight of 125 kg. We administered 1200 mg of oral acetylcysteine or matched placebo approximately 1 hour before angiography and again 1 hour after angiography. Patients were instructed to continue to take 1200 mg of acetylcysteine or matched placebo twice daily for the following 4 days for a total of 10 doses.
We collected a blood sample from each patient before the initiation of trial intravenous fluids (baseline) and at 3 to 5 days and 90 to 104 days after angiography. Among patients who did not provide a blood sample at 90 to 104 days, we continued to attempt to obtain the sample through day 180. We shipped specimens obtained from all patients at Veterans Affairs sites to a centralized trial laboratory (Advanced Bio Medical Labs), where serum creatinine was measured simultaneously in all three samples for each patient by means of an isotope dilution mass spectrometry (IDMS) traceable assay. Serum creatinine measurements of samples obtained from patients at George Institute sites were performed locally with the use of an IDMS traceable assay. We collected urine for local measurement of albumin and creatinine at the time of angiography and for pH measurement 2 to 4 hours after angiography.
TRIAL END POINTS
The primary end point was a composite of death, the need for dialysis, or a persistent increase of at least 50% from baseline in the serum creatinine level at 90 to 104 days after angiography and confirmed at subsequent testing within 14 days (defined as persistent impairment in kidney function). We ascertained death and the need for dialysis by reviewing electronic medical records and all hospitalizations within 90 days after angiography and by interviewing patients or family members. The subsequent creatinine testing was performed by means of the same method that was used in testing of the original sample.
Secondary end points were contrast-associated acute kidney injury, which was defined as an increase in serum creatinine of either at least 25% or at least 0.5 mg per deciliter (44 μmol per liter) from baseline at 3 to 5 days after angiography; death within 90 days; dialysis of any kind within 90 days; confirmed persistent kidney impairment at 90 to 104 days; hospitalization with acute coronary syndrome, heart failure, or stroke within 90 days; and hospitalization for any cause within 90 days.
STATISTICAL ANALYSIS
We used a modified intention-to-treat analysis that included all the patients who had undergone randomization and who had received the assigned trial interventions, regardless of whether they had undergone angiography. Excluded from this analysis were patients who had not received the trial interventions and had not undergone angiography. We estimated that 7680 patients would need to be enrolled for the trial to have a power of 90% to detect a decrease in the rate of the primary end point from 8.7% to 6.5% for each trial intervention, assuming a 3% loss to follow-up and no interaction between the trial interventions.12
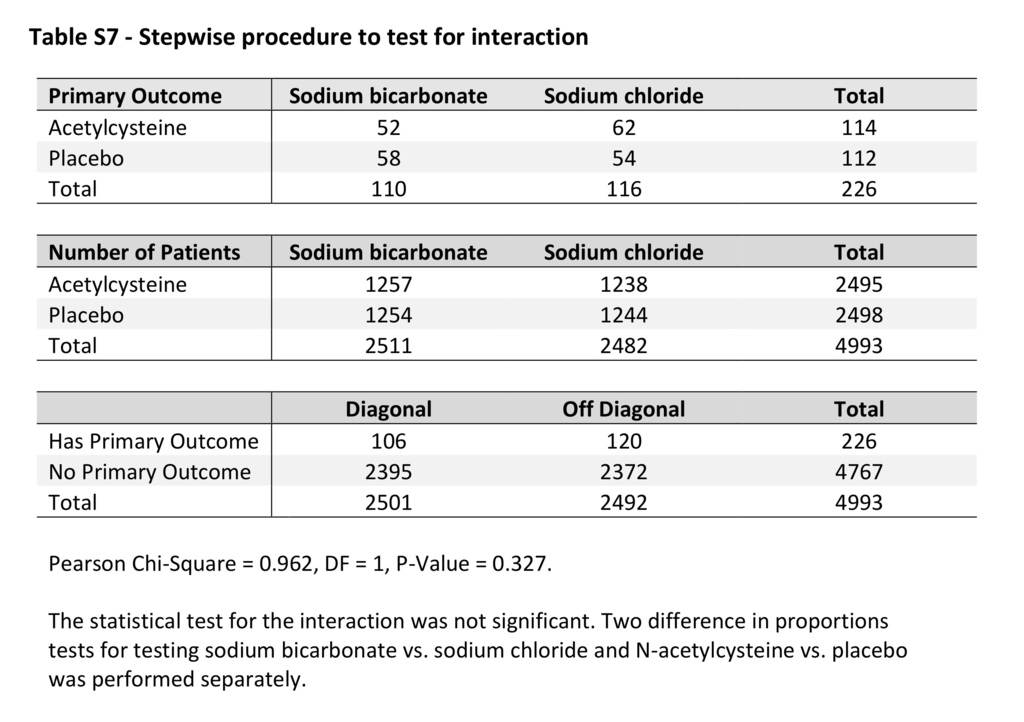
We compared baseline and procedural characteristics between the sodium bicarbonate group and the sodium chloride group and between the acetylcysteine group and the placebo group using the t-test for normally distributed continuous variables, the Wilcoxon rank-sum test for variables without a normal distribution, and the chi-square test for categorical variables. To test the interaction between sodium bicarbonate and acetylcysteine, we used a multivariable logistic-regression model that included the two randomized treatments and their interaction as parameters. In the analysis of the primary and secondary end points, a P value of less than 0.05 on the Wald chi-square test was considered to indicate statistical significance for the interaction term and to determine its inclusion or exclusion in the final logistic-regression model. We performed a stepwise procedure to confirm the results of interaction testing.
In a prespecified interim analysis, we performed the O’Brien–Fleming multiple-testing procedure after approximately 50% of the expected number of patients had been followed through 90 days. At the time of the interim analysis, we used the group sequential method to estimate the conditional power of a two-proportions test according to the rate of the primary end point in which we considered three different scenarios of accrual of new primary events across the intervention groups, with the alpha levels for each of the scenarios set at 0.048 and 0.024.14 We allowed for an overall two-sided type I error of 2.5% for each hypothesis being tested. Hence, the interim hypotheses were tested at a P value of 0.001, and the final hypotheses at a P value of 0.024. We also conducted exploratory analyses among prespecified subgroups, according to eGFR stratum, the presence of diabetes, albuminuria stratum, contrast volume, type of angiography, and country (United States vs. Australia, New Zealand, and Malaysia). Tests for all outcomes were two-sided. Statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
Results
PATIENTS
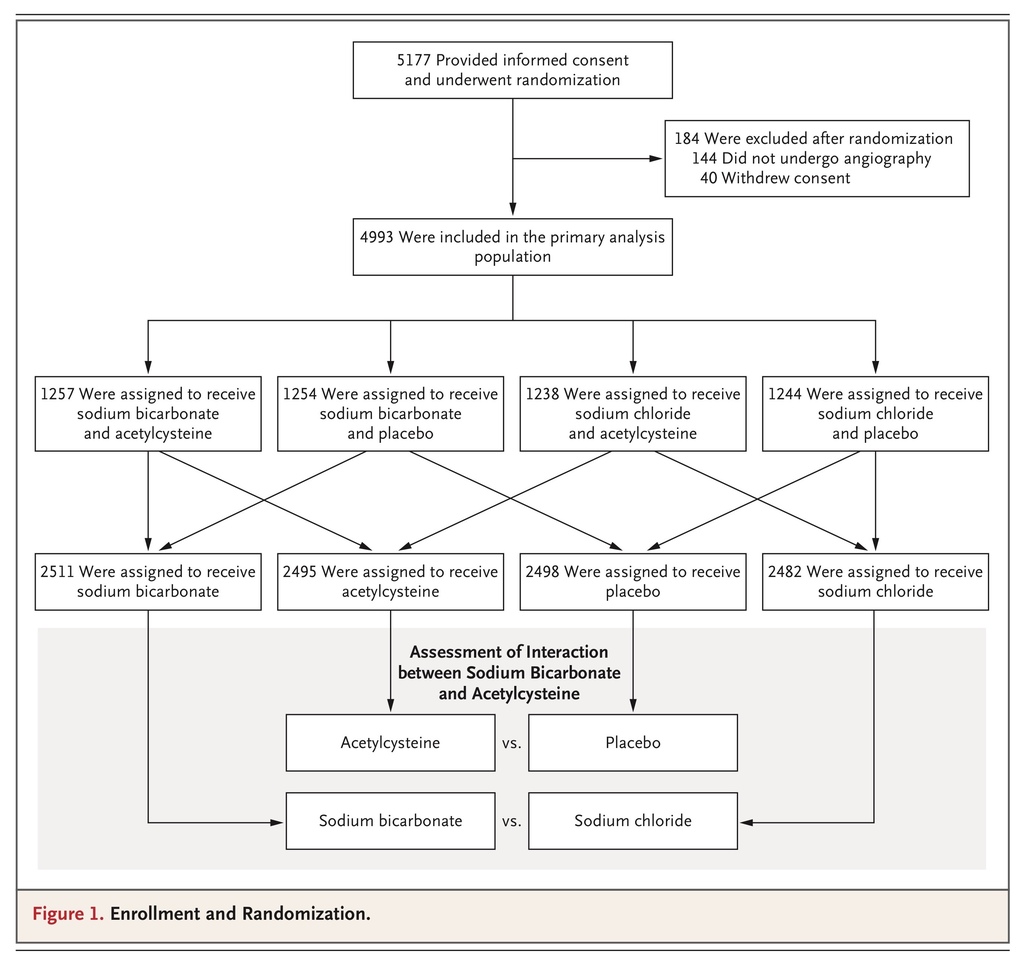
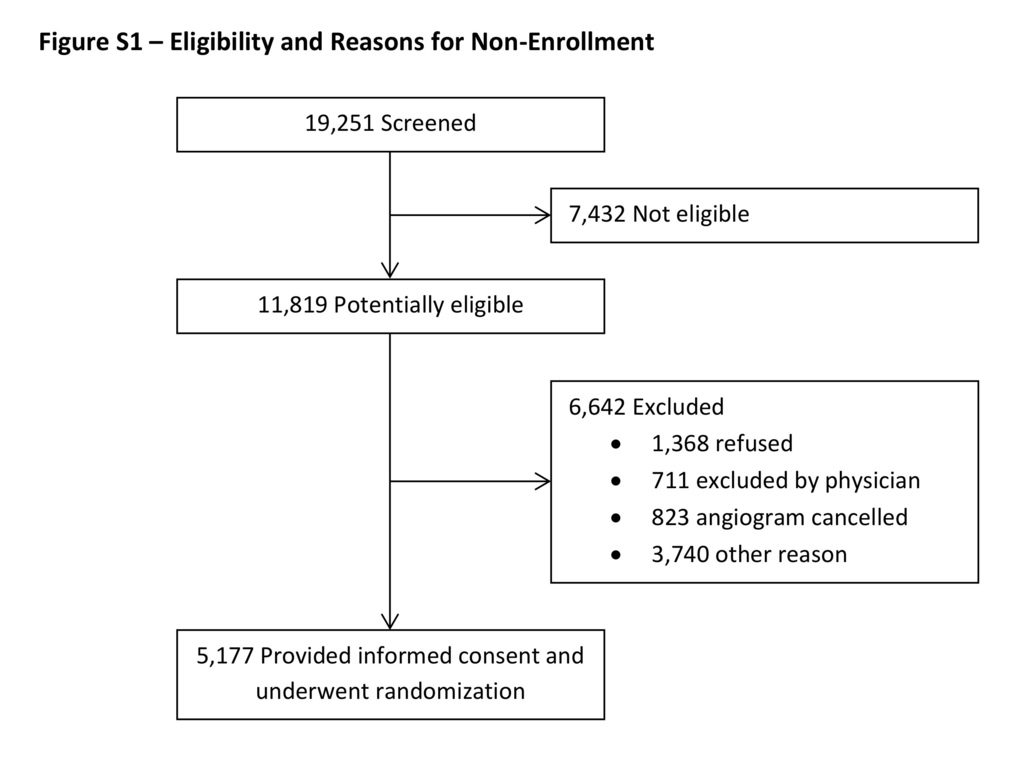
From February 2013 through March 2017, a total of 5177 patients (4441 at Veterans Affairs sites and 736 at George Institute sites) underwent randomization. A total of 184 patients (3.6%) were withdrawn after randomization (because of cancellation of angiography before receipt of the trial interventions in 144 patients and withdrawal of consent in 40), which resulted in a primary analysis group of 4993 patients; this group included 56 patients (1.1%) who had received trial interventions but had not undergone angiography. Reasons for nonenrollment of screened patients are summarized in Figure S1 in the Supplementary Appendix.
At the preplanned interim analysis, the data and safety monitoring committee recommended to the Veterans Affairs Cooperative Studies Program that enrollment in the trial be stopped on the basis of the absence of between-group difference in the rate of the primary end point for each comparison between interventions in the modified intention-to-treat analysis and results indicating that even with complete enrollment, the conditional power would be 5.2% at an alpha level of 0.024 and 11.3% at an alpha level of 0.048 if all new primary events occurred at the observed rates only in the control groups. The Veterans Affairs Cooperative Studies Program accepted this recommendation and instructed the executive committee to stop enrollment as of March 31, 2017.
BASELINE CHARACTERISTICS
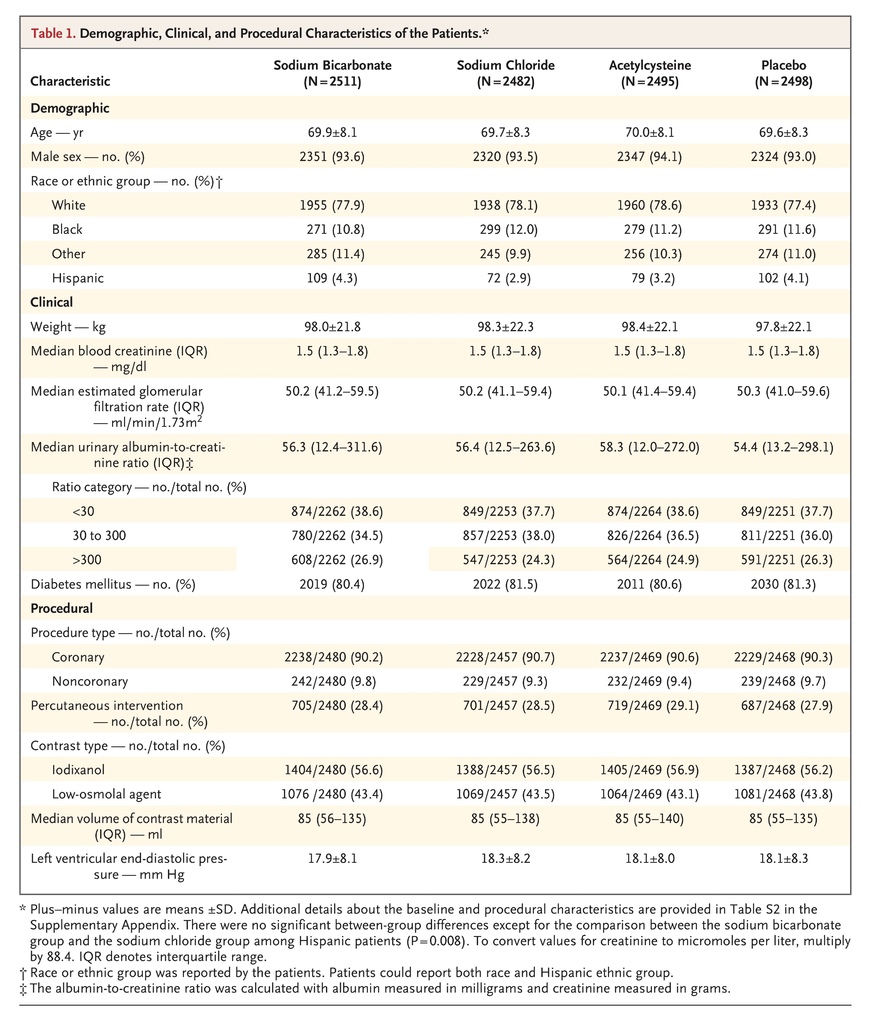
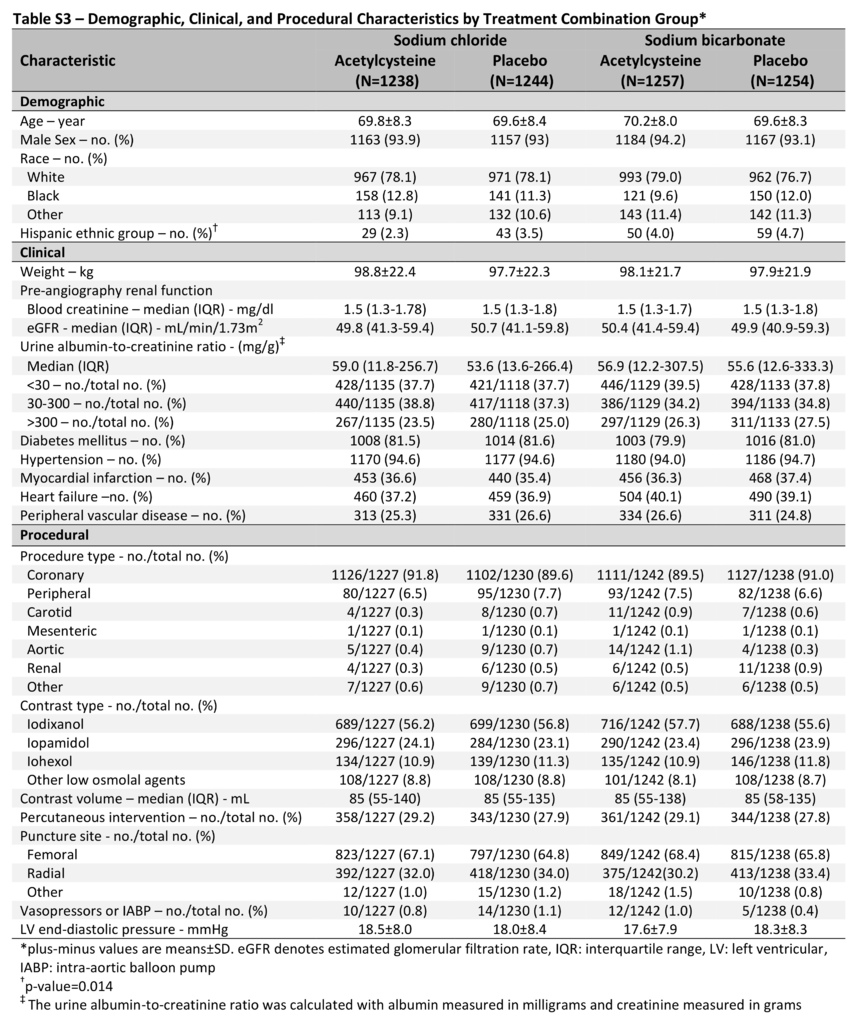
Of the 4993 patients, we randomly assigned 2511 to receive sodium bicarbonate, 2482 to receive sodium chloride, 2495 to receive acetylcysteine, and 2498 to receive placebo (Figure 1). The mean (±SD) age of the patients was 69.8±8.2 years; 4671 patients (93.6%) were men, and 4041 (80.9%) had diabetes mellitus. At baseline, the median serum creatinine level was 1.5 mg per deciliter (interquartile range, 1.3 to 1.8 [133 μmol per liter; interquartile range, 115 to 159]), and the median eGFR was 50.2 ml per minute per 1.73 m2 (interquartile range, 41.1 to 59.4). There were more Hispanic patients assigned to the sodium bicarbonate group than to the sodium chloride group (4.3% vs. 2.9%, P=0.008). There were no other significant differences in any baseline characteristics between the trial groups (Table 1, and Table S2 in the Supplementary Appendix) or across the four treatment combination groups (Table S3 in the Supplementary Appendix).
PROCEDURAL CHARACTERISTICS AND ADHERENCE TO TRIAL INTERVENTIONS
Of the 4937 patients who underwent angiography, 4466 (90.5%) underwent coronary angiography, and 471 (9.5%) underwent noncoronary angiography; 1406 (28.5%) also underwent percutaneous intervention. The median volume of contrast material that was administered was 85 ml (interquartile range, 55 to 137). Procedural characteristics were similar in the trial groups (Table 1, and Table S2 in the Supplementary Appendix) and across the four treatment combination groups (Table S3 in the Supplementary Appendix).
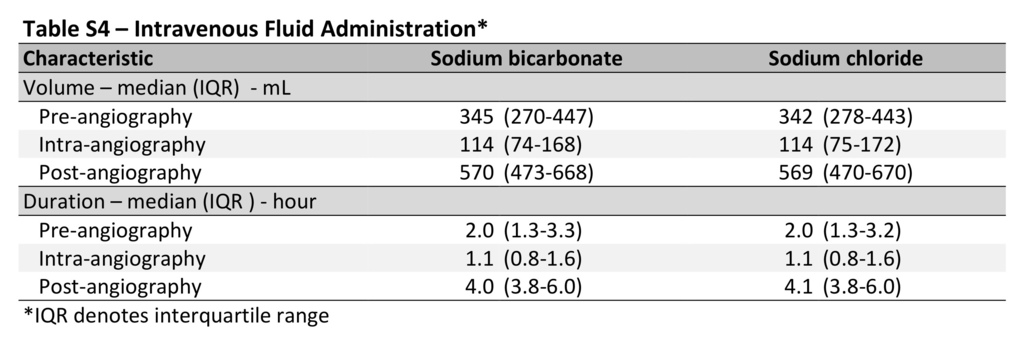
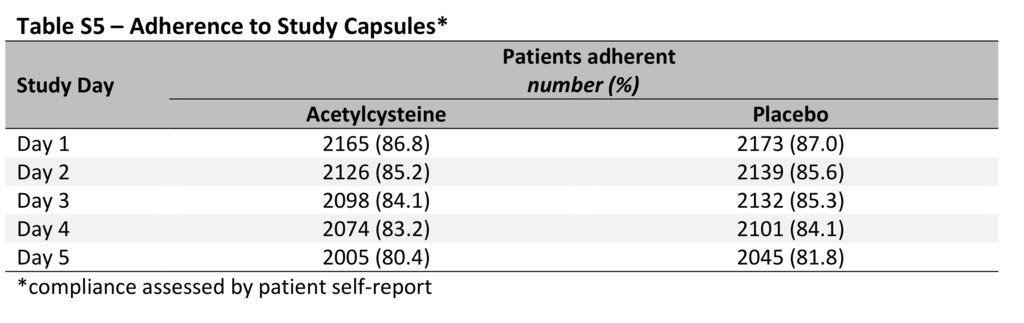
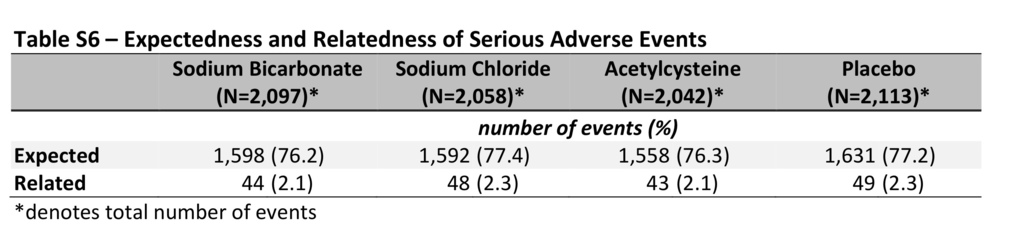
The median volume of trial intravenous fluid that was administered was 344 ml (interquartile range, 274 to 444) before angiography, 114 ml (interquartile range, 74 to 170) during angiography, and 570 ml (interquartile range, 472 to 670) after angiography; the volumes of sodium bicarbonate and sodium chloride and durations of administration were similar in the trial groups (Table S4 in the Supplementary Appendix). After angiography, the mean urine pH value was 6.7±0.8 in the sodium bicarbonate group and 6.0±0.8 in the sodium chloride group (P<0.001). Overall, 4050 of 4993 patients (81.1%) adhered to the prescribed regimen of acetylcysteine and placebo capsules, with similar rates of adherence in the two trial groups (Table S5 in the Supplementary Appendix). There were no significant differences in serious adverse events across the trial groups (Table 2, and Table S6 in the Supplementary Appendix).

PRIMARY END POINT
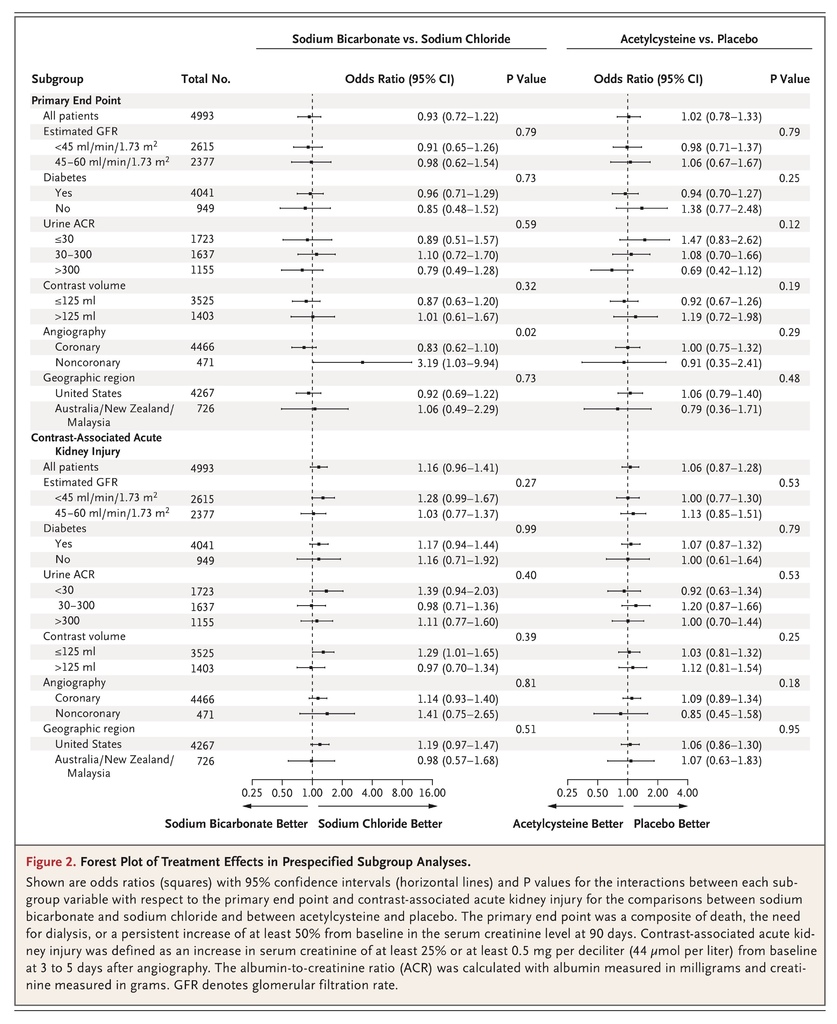
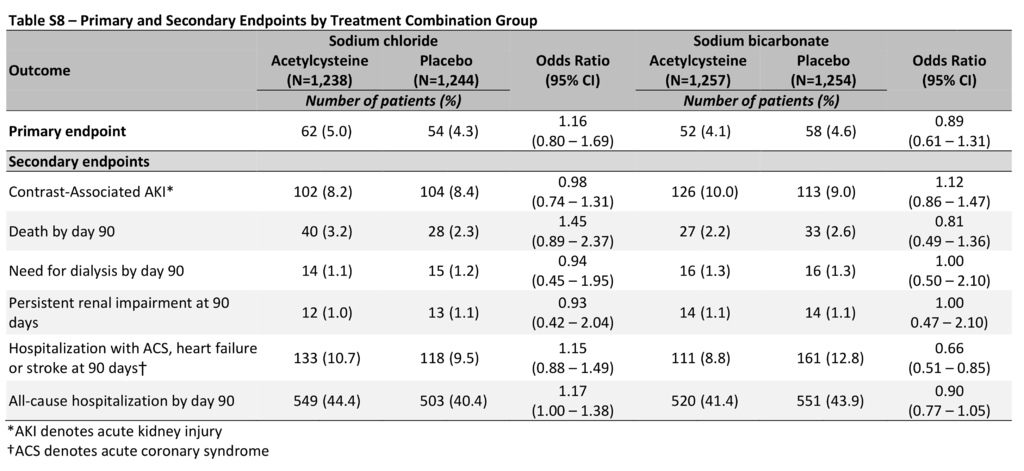
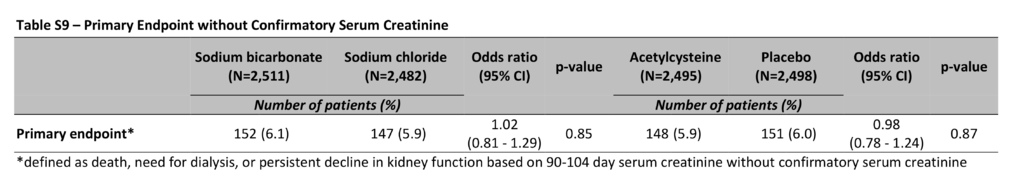
There was no significant interaction between sodium bicarbonate and acetylcysteine (P=0.33), so the interaction term was excluded from the final logistic-regression model. The absence of significant interaction was confirmed with the use of a stepwise procedure (Table S7 in the Supplementary Appendix). The primary composite end point occurred in 110 patients (4.4%) in the sodium bicarbonate group as compared with 116 patients (4.7%) in the sodium chloride group (odds ratio, 0.93; 95% confidence interval [CI], 0.72 to 1.22; P=0.62), and in 114 patients (4.6%) in the acetylcysteine group as compared with 112 (4.5%) in the placebo group (odds ratio, 1.02; 95% CI, 0.78 to 1.33; P=0.88) (Table 3). There were no significant differences in the primary composite end point in the comparison of treatment combination groups (Table S8 in the Supplementary Appendix). There were no significant differences in the primary composite end point in any prespecified subgroup in the comparison of sodium bicarbonate with sodium chloride or in the comparison of acetylcysteine with placebo, other than among the patients who had undergone noncoronary angiography, in whom sodium bicarbonate was associated with a higher risk of the primary composite end point than was sodium chloride, without adjustment for multiple comparisons (odds ratio, 3.19; 95% CI, 1.03 to 9.94) (Figure 2). There was also no benefit with either intervention when the persistent kidney impairment component of the primary end point was defined solely on the basis of the serum creatinine sample obtained at 90 to 104 days after angiography without the confirmatory sample (Table S9 in the Supplementary Appendix).
SECONDARY END POINTS
The interaction between sodium bicarbonate and acetylcysteine with respect to contrast-associated acute kidney injury was not significant (P=0.46) and was excluded from the final logistic-regression model. Contrast-associated acute kidney injury occurred in 239 patients (9.5%) in the sodium bicarbonate group as compared with 206 (8.3%) in the sodium chloride group (odds ratio, 1.16; 95% CI, 0.96 to 1.41; P=0.13) and in 228 patients (9.1%) in the acetylcysteine group as compared with 217 (8.7%) in the placebo group (odds ratio, 1.06; 95% CI, 0.87 to 1.28; P=0.58) (Table 3). There were no significant differences in contrast-associated acute kidney injury in the comparison of treatment combination groups (Table S8 in the Supplementary Appendix) or in any of the prespecified subgroups in the comparison between sodium bicarbonate and sodium chloride or in the comparison between acetylcysteine and placebo (Figure 2). There were no significant differences in the incidence of any of the other secondary end points in the comparisons between sodium bicarbonate and sodium chloride or in the comparisons between acetylcysteine and placebo (Table 3).
Discussion
In this multinational, randomized, controlled trial in patients with chronic kidney disease who were undergoing angiography, we found no benefit of intravenous sodium bicarbonate over intravenous sodium chloride or of oral acetylcysteine over oral placebo for the prevention of death, need for dialysis, or persistent kidney impairment at 90 days or for the prevention of contrast-associated acute kidney injury or other secondary end points.
Multiple previous trials and meta-analyses that have compared sodium bicarbonate with sodium chloride and evaluated acetylcysteine for the prevention of contrast-associated acute kidney injury have shown inconsistent results.5-11,15,16 Despite equipoise regarding the effectiveness of these treatments, they are widely used in clinical practice.17 However, most trials of these interventions have been underpowered.18 Our trial was stopped after the enrollment of 5177 patients of a planned cohort of 7680 (67.4%); of the patients who had undergone randomization, 4993 were included in the primary analysis. Even so, our trial population was more than twice as large as the population in the largest previous trial of acetylcysteine and much larger than the populations in all the previous trials of intravenous sodium bicarbonate.6,19 Unlike most previous trials in which the primary end point was a small increase in the blood creatinine level occurring within days after the intravascular administration of contrast material, our primary end point was a composite of serious adverse outcomes that are recognized sequelae of acute kidney injury and we analyzed contrast-associated acute kidney injury only as a secondary end point.16,20,21 Small, short-term changes in creatinine levels after the administration of contrast material are associated with progressive chronic kidney disease, cardiovascular complications, and death; however, several previous trials have not shown decreased mortality or decreased need for dialysis despite reductions in contrast-associated acute kidney injury with the use of sodium bicarbonate or of acetylcysteine.22-24 Consequently, our finding that the use of sodium bicarbonate and acetylcysteine did not reduce the incidence of our primary or secondary end points supports the strong likelihood that these interventions are not clinically effective in preventing acute kidney injury or longer-term adverse outcomes after angiography.
We restricted our study population to patients with stage 3 or 4 chronic kidney disease (eGFR of 30 to 59.9 ml per minute per 1.73 m2 for stage 3 and 15 to 29.9 ml per minute per 1.73 m2 for stage 4); those with stage 3A (eGFR of 45 to 59.9 ml per minute per 1.73 m2) were required to have diabetes mellitus, a condition that increases the risk of contrast-associated acute kidney injury in patients with impaired kidney function.25 Conversely, patients with normal or nearly normal kidney function have been included in several previous trials of these interventions, including the Acetylcysteine for Contrast-Induced Nephropathy Trial (ACT).26-29 Of the 2308 patients who were enrolled in ACT, more than half had a baseline eGFR of more than 60 ml per minute per 1.73 m2.19 Although ACT showed no benefit associated with acetylcysteine for the prevention of contrast-associated acute kidney injury, death, or the need for dialysis at 30 days, the inclusion of many patients with preserved kidney function limited the generalizability of the results among patients at higher risk for acute kidney injury and associated adverse outcomes.19
Our trial has certain limitations. First, the use of a primary end point that was assessed at 90 days created the possibility that any benefit of the interventions might have been masked by intervening events (e.g., coronary-artery bypass surgery). However, this possibility was markedly diminished by the absence of benefit with respect to contrast-associated acute kidney injury for either study intervention. Second, to assess contrast-associated acute kidney injury, we measured serum creatinine at a single time point after angiography. Consequently, patients with earlier transient decrements in kidney function could have been missed. Third, since we recruited most of the trial patients from Veterans Affairs hospitals, our population was predominantly male. Fourth, because we did not use a single protocol for the administration of intravenous fluids, all the patients did not receive identical fluid volumes. However, the volumes of fluids that were administered were similar across groups.Furthermore, our approach closely approximated clinical practice, which enhances the generalizability of the results. Fifth, many of our patients underwent diagnostic angiography with relatively small volumes of contrast material and without percutaneous interventions, which diminished the risk of contrast-associated acute kidney injury and may account for the lower-than-predicted event rates observed. Finally, by enrolling only patients who were undergoing angiography, our findings may not be generalizable to other procedures that involve the intravenous administration of iodinated contrast material, although there is no biologic reason to believe the results would not apply in such circumstances.
In conclusion, in patients with impaired kidney function who were undergoing angiography, we found that periprocedural intravenous isotonic sodium bicarbonate showed no benefit over intravenous isotonic sodium chloride with respect to the risk of major adverse kidney events, death, or acute kidney injury. In addition, we found no benefit for the oral administration of acetylcysteine over placebo in decreasing the same risks.

另外補充
Prevention of Contrast-Associated Acute Kidney Injury
Contrast-associated acute kidney injury signifies a severe and usually reversible decline in kidney function that may develop within 72 hours after intravascular administration of iodinated contrast material.1 The condition is usually defined as an increase in the serum creatinine level of more than 0.5 mg per deciliter (44 μmol per liter) or an increase of at least 25% in the level from baseline. Contrast-associated acute kidney injury occurs most commonly after contrast-enhanced computed tomography, coronary angiography, or percutaneous coronary interventions, circumstances in which the volume of contrast material that is administered is large or the administration is intraarterial.
The incidence of contrast-associated acute kidney injury varies widely, depending on well-described risk factors, such as chronic kidney disease, diabetic nephropathy, an age of more than 75 years, and congestive heart failure, and is associated with important short-term and long-term complications and increased mortality.1,2 Furthermore, subsequent long-term decline in the estimated glomerular filtration rate after an episode of contrast-associated acute kidney injury has been shown to be associated with progressive chronic kidney disease.3 Contrast-associated acute kidney injury also has economic consequences, including an increased duration of hospitalization and increased hospital costs.4
Prevention of contrast-associated acute kidney injury has centered on its multifactorial pathogenesis, which includes renal vasoconstriction and tissue hypoxia, direct cytotoxicity, increased oxidative stress, and increased blood viscosity.5 Various preventive strategies have been developed, with recent interest centering on the periprocedural administration of intravenous sodium bicarbonate or oral acetylcysteine rather than on intravenous saline hydration, which has been the standard of care since the 1990s.6 Small initial studies showed benefit from the use of sodium bicarbonate and acetylcysteine in the prevention of contrast-associated acute kidney injury.7,8 However, after the publication of these initial studies, numerous small and underpowered trials, as well as meta-analyses, raised doubt about these approaches. Indeed, there are a staggering number of small, generally less-than-informative trials in this area, with a recent literature search turning up more than 100 studies of acetylcysteine and more than 50 studies of sodium bicarbonate. Such conflicting data have left clinicians with confusing choices about the most effective preventive approach.
In this issue of the Journal, Weisbord et al.9 report the results of the Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial, which compared intravenous saline, intravenous sodium bicarbonate, and oral acetylcysteine for the prevention of the primary end point, which was a composite of death, the need for dialysis, or a persistent increase in the serum creatinine level of at least 50% from baseline at 90 days after the procedure. Contrast-associated acute kidney injury was a secondary end point. This multicenter, randomized, controlled trial enrolled 5177 patients in 2-by-2 factorial design to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride and 5 days of oral acetylcysteine or oral placebo. Patients were at moderate risk for contrast-associated acute kidney injury (defined as chronic kidney disease stage 3 or worse, with or without diabetes), and the rate of acute kidney injury of approximately 9% in the trial was consistent with this risk stratification. Higher-risk patients, such as those undergoing emergency procedures, were not included in the trial, and the majority of the studies were diagnostic, not interventional. The trial was stopped early for futility after a prespecified interim analysis showed that there was no between-group difference in the primary end point and that complete enrollment was not likely to alter the results. Thus, the trial investigators concluded that there was no additional benefit of sodium bicarbonate over saline, no benefit of acetylcysteine over placebo, and no interactions between these therapies. Subgroup analyses across several different parameters also showed no benefits.
It is important to note that the authors used a primary end point that reflects the sequelae of contrast-associated acute kidney injury rather than focusing on small changes in the serum creatinine level. Their strategy is more clinically relevant, and the finding that neither the primary nor the secondary end point was affected by these therapies strongly suggests that these interventions have no clinical efficacy over saline.
The trial has some limitations. One of the greatest is that the protocol allowed for wide discretion in the amount of intravenous fluid that was administered to patients. Furthermore, clinicians were aware of the patients’ clinical characteristics. Thus, one could wonder whether higher-risk patients received more fluids for longer durations, thereby biasing the results. However, the volume of the administered fluid was similar in all groups. In addition, patients at the highest risk for contrast-associated acute kidney injury, such as those with diabetic nephropathy and those receiving large volumes of contrast material for interventional procedures, constituted a very small percentage of the enrolled patients. Despite these and other limitations, this large, well-designed trial should prompt clinicians to focus on the periprocedural use of intravenous saline and abandon the use of sodium bicarbonate and acetylcysteine for the prevention of contrast-associated acute kidney injury. The results of the PRESERVE trial remind us that small, underpowered, single-center studies may be regarded with skepticism until the findings can be confirmed in well-designed, randomized, controlled clinical trials.























 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 