March 29, 2007 — The manufacturers of pergolide in its brand-name
(Permax; Valeant Pharmaceuticals) and generic forms have agreed to take
it off the market after a pair of studies implicated the dopamine agonist in
causing serious heart valve damage, the US Food and Drug Administration (FDA)
has announced [1].
Two case control studies published in the January 4,
2007 issue of the New England Journal of Medicine (NEJM) found
significantly increased rates of valvular dysfunction in patients with
Parkinson's disease taking pergolide and another dopamine agonist, cabergoline
[2,3]. As reported at the time by heartwire , the findings were
consistent with abundant clinical and mechanistic evidence that activators of
the serotonin receptor 5-hydroxytryptamine 2B (5-HT2B), such as
pergolide and cabergoline, cause a histologically distinct form of fibrotic
valvulopathy. Cabergoline is approved in the US for the treatment of
hyperprolactinemic disorders at doses much lower and safer than those used in
Parkinson's disease, according to the FDA statement, which was published online
today.
Those NEJM studies, along with the availability of other
dopamine agonists for Parkinson's disease, have led pergolide manufacturers to
withdraw the drug voluntarily, which will have a delayed effect on pharmacy
inventories, according to the FDA: "This delay will allow time for healthcare
professionals and patients to discuss appropriate treatment options and to
change treatments." Manufacturers of the generic products, the agency notes,
include Par Pharmaceutical Companies and Teva Pharmaceutical Industries.
The FDA recommends that patients currently taking pergolide contact
their healthcare providers to discuss alternative treatments but not go off the
drug on their own. Clinicians are urged to assess alternatives and, if
necessary, substitute another dopamine agonist; others remaining on the market
have not been linked to valve disease. Current pergolide therapy should not be
stopped abruptly but should be gradually tapered, the agency cautions.
- http://www.fda.gov/cder/drug/advisory/pergolide.htm
- Schade R, Andersohn F, Suissa S, et al. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 2007; 356:29-38.
- Zanettini R, Antonini A, Gatto G, et al. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med 2007; 356:39-46.
順便幫大家回憶一下pergolide的機轉
Pergolide mesylate, a semisynthetic ergot derivative, is a dopamine receptor agonist. In in vitro binding studies, pergolide demonstrated high binding affinity for dopamine D2 and D3 and lesser affinity for D1 receptors. Pergolide binds with high affinity to α2-adrenergic receptors and with moderate affinity to α1- adrenergic or serotonin (5-hydroxytryptamine [5-HT]) receptors.
The exact mechanism(s) of action of pergolide has not been fully elucidated. There is considerable evidence that manifestations of parkinsonian syndrome, regardless of the cause of the syndrome, are related to depletion of dopamine in the corpus striatum. While levodopa is believed to act principally by increasing dopamine concentration in the brain, pergolide appears to act by directly stimulating postsynaptic dopamine receptors in the corpus striatum.
Pergolide reduces serum prolactin concentrations by inhibiting release of prolactin from the anterior pituitary gland. This effect on hypothalamic/pituitary function has been attributed to the drug’s agonist activity at D2 receptors. Pergolide causes transient increases in serum somatotropin (growth hormone) concentrations and decreases in serum luteinizing hormone (LH) concentrations.
Limited information is available on the pharmacokinetics of pergolide mesylate. The drug undergoes extensive enterohepatic metabolism; the absolute oral bioavailability is low (about 20%). Pergolide is metabolized to at least 10 metabolites; 2 of these metabolites (pergolide sulfoxide and pergolide sulfone) have dopamine agonist activity.
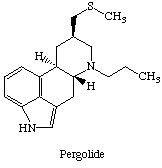
最後,讓大家回憶一下他的結構:





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 