Emily M. Ambizas, PharmD, CGP
Associate Clinical Professor
St. John’s University, College of Pharmacy & Allied Health Professions
Queens, New York
Clinical Specialist, Rite Aid Pharmacy
Whitestone, New York
Priti N. Patel, PharmD, BCPS
Assistant Clinical Professor
Director, Drug Information Center
St. John’s University, College of Pharmacy & Allied Health Professions
Queens, New York
4/19/2010
US Pharm. 2010;35(4):34-41.
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a common ophthalmologic disorder experienced by many people and can be a reason for pharmacist consultation.1 It is a condition that can adversely affect a patient’s quality of life, impacting daily activities such as reading, using a computer, driving, and watching television.2 The prevalence of DED varies, but it has been estimated to affect up to 33% of the population, although this number may be underreported.1,3
In 2007, the International Dry Eye Workshop (DEWS) acknowledged the multifactorial nature of the disease and defined DED as “a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear fill and inflammation of the ocular surface.”4
Etiology
The DEWS recommendations classified the etiology of DED into two categories: aqueous tear-deficient and evaporative.4
Aqueous tear-deficient dry eye is due to decreased volume and production of tears. This may be caused by Sjögren’s syndrome or stem from non-Sjögren’s causes. Sjögren’s syndrome, an autoimmune disease, attacks the lacrimal and salivary glands, leading to dry mouth and dry eyes. Non-Sjögren’s dry eye is caused by lacrimal dysfunctions, including lacrimal deficiencies, obstruction of the lacrimal gland ducts, and reflex hyposecretion.4
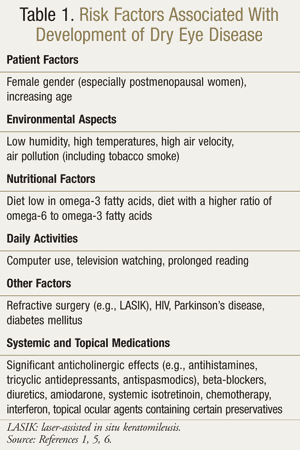
Evaporative dry eye is due to increased tear evaporation in the presence of normal lacrimal secretion. The cause of this may be described as intrinsic or extrinsic. Intrinsic causes include meibomian gland dysfunction (the most common cause of evaporative dry eye), eyelid aperture disorders or lid/globe incongruity, and decreased blink rate. Extrinsic causes include ocular surface disorders due to vitamin A deficiency, use of topical anesthetics and preservatives, contact lens wear, and allergic conjunctivitis.4TABLE 1 lists risk factors associated with DED.1,5,6
Symptoms
Patients with DED typically experience ocular discomfort, photosensitivity, and blurry vision. Ocular discomfort is described as a dry, scratchy, gritty, or sandy feeling, as well as foreign body sensations, irritation, soreness or pain, burning, itching, and ocular fatigue. Patients may also complain about mucous discharge, contact lens intolerance, and red eyes. Initially, patients may present with excessive tearing due to corneal irritation, causing reflex tearing.6,7 The onset of symptoms is usually gradual, bilateral, and chronic; symptoms typically become more bothersome later in the day and are intensified by various environmental factors (TABLE 1).6
Diagnosis
DED may be diagnosed by an eye care professional, although the diagnosis may be challenging due to the lack of uniform criteria.6,8,9
A combination of tests, both subjective and objective, are used in the evaluation of DED. Objective clinical measures include assessing tear film instability, ocular surface damage, and aqueous tear flow.6,8,10 Tear osmolarity measurement has been suggested to be highly diagnostic for dry eye, as it is testing for a factor that is directly related to the cause.8,10 Questionnaires are used to determine the severity of symptoms, effect on daily activities, and impact on quality of life. However, questionnaires should never be used alone when diagnosing DED and should always be used in combination with objective data collected through the use of diagnostic tests.
Treatment Options
The goals of treatment for DED are to reduce ocular discomfort, improve quality of life, and return the ocular surface and tear film to their normal states, preventing further damage to the ocular tissue and cornea. Treatment approaches vary; there are many nonpharmacologic and pharmacologic therapies to choose from.11 Ultimately, the underlying cause should be identified and corrected. The mainstay of treatment is to lubricate the eye with artificial tear supplements.1,12
Nonpharmacologic Treatment: Educating patients about the avoidance of environmental factors can help improve the symptoms of DED. This can be accomplished by advising the patient to avoid wind, smoke, and dust, and to use a humidifier. Taking breaks from reading and computer use and increasing blink frequency can also help. The patient may also try washing the eyelid with a mild soap or baby shampoo to decrease bacterial colonization, and applying warm compresses to reduce evaporative loss. Exacerbating medications may be discontinued if appropriate and feasible. These interventions will alleviate symptoms but will not completely control them; the patient will need to utilize topical or systemic medications to adequately manage DED.12,13
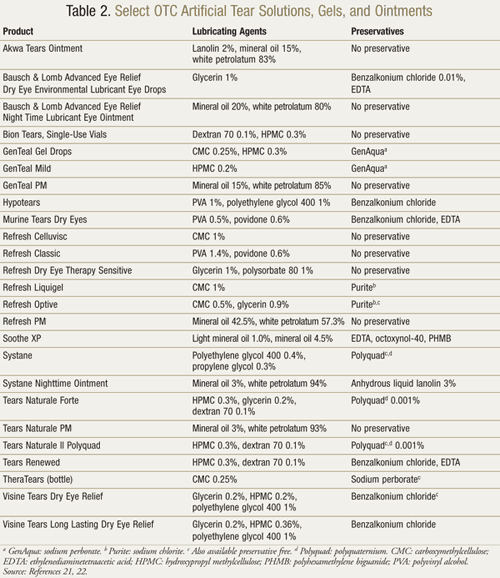
OTC Products: The numerous OTC products available may cause confusion (TABLE 2). The aspects of the differing formulations that pharmacists should be aware of include the ingredients and the presence of preservatives.
Artificial tears may contain any number of ingredients to increase viscosity, including carboxymethylcellulose, polyethylene glycol, hydroxypropyl methylcellulose, glycerin, and polyvinyl alcohol. Increased viscosity of the product will increase tear retention time.13Viscosity-enhancing agents may protect the epithelium of the eye by coating the surface.11 Oil-containing eye drops also lubricate the eye. These may decrease tear evaporation by restoring the lipid layer of tear film.11 Ophthalmic ointments are not considered artificial tears and are typically reserved for overnight use due to their high viscosity and increased chance of blurring the vision.13Ointments are formulated with white petrolatum and mineral oil, and some also contain the potentially irritating agent lanolin. Electrolyte ingredients can help treat the surface of the eye. Most notably, potassium helps maintain corneal thickness, while bicarbonate helps maintain normal epithelial structure.11
Preservatives are included in many ophthalmic formulations to decrease the chance of bacterial contamination during product application and to increase shelf life of the product. The FDA requires multidose ophthalmic agents to contain preservatives.11Single-use containers of ophthalmic agents typically do not contain preservatives.
Preservatives can exacerbate ocular inflammation of dry eye. The DEWS recommendations state that for patients with moderate-to-severe dry eye who require frequent application of ocular lubricant, the absence of preservative is a more important consideration than the active lubricating agent.11
Benzalkonium chloride, a common preservative found in ophthalmic agents, has toxic effects on the eye when used topically and is not recommended for patients with severe DED. The DEWS recommendations state that benzalkonium chloride is generally well tolerated when used less than 4 to 6 times per day.11
Ethylenediaminetetraacetic acid (EDTA) is another preservative frequently found in ophthalmic products, and it may also cause irritation in patients who use the drops several times per day.11 Newer preservatives include polyquaternium-1, sodium chlorite, and sodium perborate. These were developed as alternatives to benzalkonium chloride and EDTA and are less irritating because they dissociate upon contact with the ocular surface and therefore do not spend time on the surface as the older preservatives do.11
Since osmolarity of tear film in dry eye patients is increased, hypo-osmotic products have been created (e.g., Hypotears, TheraTears). These products may help counteract the proinflammatory nature of the high osmolarity tear film. Products with higher colloidal osmolarity may help restore appropriate water transport across ocular membranes. Compatible solutes, including glycerin and levocarnitine, have been incorporated into products. These are taken up by ocular epithelial cells without disrupting their normal functions and help to increase intracellular osmolarity.11
A list of representative artificial tear products and ocular lubricants is given in TABLE 2.
Prescription Products: Patients who do not respond to OTC treatments or require multiple doses of OTC treatments daily may benefit from prescription products.
FreshKote is a prescription product that is FDA approved as an ocular lubricant for moderate-to-severe dry eye. This product is a formulation of polyvinyl pyrrolidone 2.0%, polyvinyl alcohol (87% hydrolyzed) 0.9%, polyvinyl alcohol (99% hydrolyzed) 1.8%, and Amisol Clear. Amisol Clear is a proprietary ingredient that is claimed to stabilize the lipid layer of tear film.14 This product is preserved with EDTA and polixetonium.
Cyclosporine topical emulsion 0.05% (Restasis) was approved in 2003 for the treatment of dry eye.15 Cyclosporine reduces T-cell activation, which can improve ocular surface health and decrease lacrimal gland inflammation. Cyclosporine also decreases cytokine production. This product is unique in that it is FDA approved to increase tear production, not just to lubricate the eye. Restasis is contraindicated in patients with active ocular infections and in patients with hypersensitivity to any ingredients. Restasis should be used as 1 drop in each eye twice daily, 12 hours apart. It can be used along with artificial tears, provided that the two are administered at least 15 minutes apart. Common adverse events include burning and stinging upon instillation, ocular discharge, eye pain, pruritus, foreign body sensation, and blurring of vision.15
Oral Supplements: Studies have shown that oral supplementation with omega-3 fatty acids decreases the likelihood of a woman experiencing dry eye.16 Supplementation with omega-3 fatty acids has also been shown to be useful in the treatment of dry eye. Omega-3 fatty acids can help restore the lipid layer of tear film, decrease inflammation, and increase tear production.13,17
Other Treatments: If the OTC and prescription products fail to achieve adequate ocular comfort for the patient, moisture chamber spectacles, contact lenses, tear stimulation via secretagogues, topical ocular corticosteroids, and various surgical techniques may be available through the care of an ophthalmologist or other eye care provider.11
Helping Patients
Depending on the severity of the patient’s case, a pharmacist may recommend a variety of products. Patients suffering from mild DED will have episodic occurrences usually due to environmental stress. Severe DED is seen in those patients who persistently experience symptoms, which can be disabling, and may have possible damage to the ocular surface.1,4 For mild cases of DED, using an artificial tear product 1 to 2 times a day is recommended. For more severe cases, pharmacists can recommend a product to be used 3 to 4 times daily.18 When recommending a product, pharmacists should remember that patients who use eye drops for dry eye frequently throughout the day would benefit from formulations that are either preservative free or include the less irritating preservatives polyquaternium-1, sodium chlorite, and sodium perborate.
Artificial tear preparations with higher viscosity tend to have longer-lasting effects, as the products stay on the eye for a longer period of time. These products, however, also cause buildup around the eye, and for cosmetic reasons may not be preferred by patients. Patients should also be instructed on lifestyle modifications and nonpharmacologic treatments as previously discussed.
Pharmacists should also be aware that elderly patients may have trouble instilling eye drops with smaller unit-dose systems.19 For such patients, larger, easier-to-use bottles may help. Visine Pure Tears No Mess Single-Drop Dispenser is a preservative-free product designed to instill eye drops without contamination and may be useful in patients who have difficulties with manual dexterity.20
It is important for pharmacists to counsel patients on proper administration of the various ophthalmic products. A step-by-step guide should be provided to patients:
• Wash hands and areas of the face around the eyes before use
• Tilt head back
• While grasping the lower eyelid, pull it away from the eye to form a pouch
• While looking up, place a single drop into the eye
• Close the eye and gently apply pressure to the tear duct
• Blot excess solution.
If an ointment is needed, the patient should place 1/4 to 1/2 inch of ointment inside the lower eyelid and close eyes gently for 2 minutes. If more than one medication is needed, wait at least 5 minutes between products. If a suspension ophthalmic product is needed, remind the patient to shake it well prior to use and to use this particular product last. In addition, patients should use ophthalmic drops at least 10 minutes prior to the use of ointments.18
Pharmacists should inform patients that although these products may relieve symptoms, they will not cure DED. If one product does not produce a response, the patient may try several others. If the patient does not feel any relief after using different products, he or she should be referred to an eye care specialist.
In addition, if patients present with any of the following conditions, they should not attempt self-treatment and should be referred to a specialist: eye pain, blurred vision, light sensitivity, chemical exposure, symptoms persisting for more than 72 hours following the use of OTC products, history of contact lens wear, red eye, blunt trauma to the eye, and exposure to heat.18
Conclusion
Dry eye disease is a common disorder experienced by many people, and it is a frequent cause of patient visits to pharmacies for OTC treatments. Pharmacists should be familiar with the various products available in order to assist patients in choosing an appropriate product.
REFERENCES
1. Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405-412.
2. Miljanovic B, Dana R, Sullivan DA, et al. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409-415.
3. Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care.
4. International Dry Eye Workshop (DEWS). The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul Surface.
5. International Dry Eye Workshop (DEWS). The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye Workshop. Ocul Surface. 2007;5:93-107.
6. Perry HD. Dry eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care.
7. Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surface.
8. International Dry Eye Workshop (DEWS). Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye Workshop. Ocul Surface. 2007;5:108-152.
9. Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol.
10. Srinivasan S, Nichols KK. Collecting tear osmolarity measurements in the diagnosis of dry eye. Expert Rev Ophthamol. 2009;4:451-453.
11. International Dry Eye Workshop (DEWS). Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye Workshop. Ocul Surface.
12. Foulks GN. Treatment of dry eye disease by the non-ophthalmologist. Rheum Dis Clin N Am.
13. Lemp MA. Management of dry eye disease. Am J Manag Care. 2008;14(suppl 3):S88-S101.
14. FreshKote package insert. North Little Rock, AR: Focus Laboratories; October 2005.
15. Restasis (cyclosporine ophthalmic emulsion 0.05%) package insert. Irvine, CA: Allergan; January 2009.
16. Miljanovic B, Trivedi KA, Dana MR, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887-893.
17. Roncone M, Bartlett H, Eperjesi F. Essential fatty acids for dry eye: a review. Cont Lens Anterior Eye.
18. Fiscella RG, Jensen MK. Ophthalmic disorders. In: Berardi RR, Ferreri SP, Hume AL, et al. eds. Handbook of Nonprescription Drugs. 16th ed. Washington, DC: American Pharmacists Association; 2009:519-543.
19. Foulks GN. Pharmacological management of dry eye in the elderly patient. Drugs Aging.
20. Visine Pure Tears. www.visine.com/product-visine- 2008;14(suppl 3):S102-S106. 2007;5:75-92. 2008;14(suppl 3):S79-S87. 2009;7:199-211. 2008;146:350-356. 2007;5:163-178. 2008;34:987-1000. 2010;33:49-54. 2008;25:105-118. pure-tears.jsp. Accessed March 23, 2010.
21. Various products. www.drugstore.com. Accessed March 23, 2010.
22. Dry eyes drug treatment. Artificial tears. The Eye Digest. www.agingeye.net/dryeyes/dryeyesdrugtreatment.php. Accessed March 3, 2010.
其他相關文章:
bromhexin用於乾襙症候群( Sjögren's syndrome)





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 