Mohammad Yawar Yakoob, MBBS; Yasir Pervez Khan, MBBS; Zulfiqar Ahmed Bhutta, MB, BS, PhD, FRCP, FRCPCH, FCPS, FAAP
Abstract and Introduction
Abstract
Deficiency of vitamins and minerals, collectively known as micronutrients, during pregnancy can have important adverse effects on maternal and birth outcomes. Evidence-based nutrition interventions can make a difference and potentially avert these outcomes. Iron supplementation has been shown to improve maternal mean hemoglobin concentration at term and reduce the risk of anemia. Zinc supplementation has been shown to result in a small but significant reduction in preterm births. A cluster-randomized study in Nepal showed a 40% reduction in maternal mortality up to 12 weeks postpartum with weekly vitamin A and 49% biweekly β-carotene supplementation but subsequent large studies in Bangladesh and Ghana have failed to demonstrate any impact on mortality. Maternal vitamin A supplementation has no role in preventing mother-to-child transmission of HIV in HIV-infected pregnant women. Periconceptional folic acid supplementation reduces the risk of neural tube defects, while supplementation with vitamin D reduces the incidence of neonatal hypocalcemia with no impact on craniotabes. Iodine supplementation during pregnancy has also been suggested to reduce the risk of perinatal and infant mortality, and the risk of endemic cretinism at 4 years of age. Calcium supplementation reduced the risk of preeclampsia in women with low baseline calcium dietary intake, while magnesium supplementation has been associated with a lower frequency of preterm births and adverse neurodevelopmental outcomes in childhood. Other vitamins and minerals, such as vitamins B, C and E, copper and selenium, have been associated with fetal development, but their impact on pregnancy outcomes is not clear. Given such widespread maternal vitamin and mineral deficiencies, it is logical to consider supplementation with multiple micronutrient preparations in pregnancy. The clinical benefits of such an approach over single-nutrient supplements are unclear, and this article explores the current concepts, evidence and limitations of maternal multiple-micronutrient supplementation.
Introduction
Undernutrition during the reproductive age group and pregnancy remains a major problem in countries where women usually do not have equitable access to food, education and healthcare.[101] Many women are undernourished at birth, stunted during childhood, become pregnant at adolescence, and are underfed and overworked during pregnancy and lactation, resulting in nutritional deficiencies.[102] Most women living in developing countries experience various biological and social stresses that, combined with other factors, increase the risk of malnutrition throughout life. These include: poverty, lack of purchasing power, food insecurity and inadequate diets, recurrent infections, poor healthcare, heavy work burdens, gender inequities and limited general knowledge about appropriate nutritional practices. These factors are compounded by high fertility rates, repeated pregnancies and short intervals between pregnancies.[1] Undernutrition undermines the woman's ability to survive childbirth and give birth to healthy children, translating into lost lives of mothers and their infants.[102] Nutrient deficiencies, whether clinical or subclinical, can also potentially affect fetal growth, cognition and future reproductive performance.[2] In the developing world, maternal and fetal undernutrition impairs their contribution to their families and communities, as well as their productivity and income-generating capacity.[102] It has been shown that maternal undernutrition reduces placental-fetal blood flow and retards fetal growth in both domestic animals and humans.[3,4] An altered intrauterine nutritional environment affects expression of the fetal genome, a phenomenon termed 'fetal programming'.[5] This impact of maternal nutritional status on fetal programming and genetic imprinting is associated with disease in later life, such as coronary heart disease and stroke, and the associated conditions such as hypertension and non-insulin-dependent diabetes. People who have low growth rates in utero also cannot withstand the stress of becoming obese as adults.[4]
Burden of Maternal Micronutrient Deficiencies and Evidence Base for Interventions
Nutritional deficiencies are widely prevalent globally and contribute significantly to high rates of morbidity and mortality among mothers and their infants and children in developing countries. The nutritional status of a woman before and during pregnancy is important for a healthy pregnancy outcome. The prevalence of maternal undernutrition - that is, a BMI of less than 18.5 kg/m2 - ranges from 10 to 19% in most countries.[6] More than 20% of women in sub-Saharan Africa, Southcentral and Southeastern Asia, and Yemen have a BMI of less then 18.5 kg/m2.[6] In India, Bangladesh and Eritrea, 40% of women have a low BMI, which has adverse effects on pregnancy outcomes and increases the risk of infant mortality.[6] Malnutrition among women manifests itself at the macronutrient and/or the micronutrient level. More than 40% of pregnant women around the world are anemic, most of which is due to iron deficiency.[103] Iron, therefore, contributes to the largest prevalence of micronutrient deficiencies. Other important deficiencies include iodine, zinc, vitamin A, folic acid and vitamin B complex, including thiamine, riboflavin and B12.[7]
There is a critical window of opportunity during the early prenatal period while the fetus is still growing to prevent undernutrition during pregnancy and its effects on maternal and child health. Evidence-based nutrition interventions can make a difference to short-term outcomes, and also offer the best opportunity for long-term growth and development. These interventions include strategies to improve maternal nutrition before and during pregnancy, with appropriate micronutrient interventions. Although iron-deficiency anemia is recognized as an important risk factor for maternal and perinatal mortality globally, the contribution of other micronutrient deficiencies to adverse outcomes of pregnancy is less clear. However, emerging evidence suggests that micronutrients such as vitamin B12, folic acid, vitamin D and selenium may also be important for maternal, infant and child outcomes.[8-10]
Iron
Iron deficiency and iron-deficiency anemia are major public health problems, affecting an estimated 30% of the world's population, mostly women of reproductive age.[102] The major clinical manifestation of iron deficiency is anemia or a low-blood hemoglobin concentration. Anemia affects 41.8% of pregnant women in the world and is a risk factor for maternal morbidity and mortality.[103] In a study conducted in India, 87% of pregnant women were found to be anemic.[11] The prevalence in other South Asian countries varies: Bangladesh 77%, Bhutan 59%, Nepal 65% and Sri Lanka 60%.[2] Iron deficiency, resulting in anemia, is highly prevalent in pregnant women, and increased requirements in pregnancy are often not met even by changes in diet. During pregnancy, iron requirements increase substantially due to increased requirements by the placenta and the fetus, and is further compounded by blood loss at delivery. Studies have shown that iron deficiency increases the rate of premature delivery and perinatal mortality.[12] Hemorrhage remains a leading cause of maternal death in developing countries, accounting for approximately 25% of all maternal deaths,[13] and iron deficiency is recognized to increase the risk of mortality among anemic women due to hemorrhage and infections.
Several UN agencies recommend the universal distribution of iron-folic acid supplements to pregnant women in the developing world. The evidence base for the effectiveness of iron supplementation in pregnancy is strong. A review by Pena-Rosas and Viteri on preventive iron or iron and folic acid supplementation during pregnancy included 49 trials involving 23,200 women.[14] Daily oral iron supplementation resulted in a significantly higher maternal mean hemoglobin (Hb) concentration (mean difference [MD]: 8.83; 95% CI: 6.55-11.11) (Figure 1) and a reduced risk of anemia in mothers at term (relative risk [RR]: 0.27; 95% CI: 0.17-0.42), compared with no intervention or placebo administration. There was also reduced risk of iron-deficiency anemia, a significantly higher risk of hemoconcentration at term and also a higher mean Hb concentration within 1 month postpartum. The benefits to the infant included a significant increase in birth length (MD: 0.38 cm; 95% CI: 0.10-0.65) and higher mean ferritin concentrations at 3 and 6 months of age. However, there was no significant direct evidence of benefits on maternal outcomes such as maternal mortality, severe anemia at term, preeclampsia, antepartum hemorrhage and postpartum hemorrhage, but few studies were powered for these effects. Similarly, infant outcomes such as perinatal death, low birthweight, small-for-gestational age, premature delivery and Hb concentrations at 3 and 6 months were not statistically different. Similar results were achieved for iron plus folic acid supplementation versus no intervention or placebo. There was a significantly higher mean Hb maternal concentration and a reduced risk of anemia at term. Hb levels within 1 month postpartum were also greater in the intervention group. There was also a reduced risk of small-for-gestational age babies, and a significantly greater birth length and mean birthweight in the iron-folate group. There was no difference in maternal iron deficiency at term or adverse maternal and infant outcomes between the two groups. Iron and folic acid supplementation during pregnancy also resulted in a significant 31% decreased risk of death in children occurring from birth to 7 years of age compared with controls receiving vitamin A only (hazard ratio [HR]: 0.69; 95% CI: 0.49-0.99).[15]
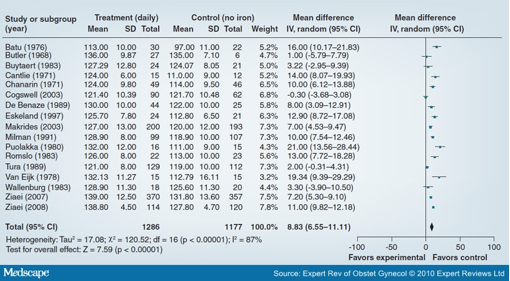
Figure 1. Effect of daily iron alone versus no intervention/placebo on maternal hemoglobin concentration at term. SD: Standard deviation. Data from [14].
Zinc
While definite data on the prevalence of zinc deficiency are hard to come by, several studies in the literature highlight the likelihood of widespread mild-to-moderate zinc deficiency in pregnant women.[2] The prevalence of zinc deficiency in developing countries is probably similar to that of nutritional iron deficiency, as the same dietary pattern induces both, with a high prevalence in South Asia, most of sub-Saharan Africa and parts of Central and South America.[6] Caulfield et al. have stated that 82% of pregnant women worldwide are likely to have inadequate usual intakes of zinc.[16] Zinc plays a role in a large number of metabolic synthetic reactions. During periods of rapid growth and higher micronutrient requirements, such as infancy, adolescence and late pregnancy, girls and women are most susceptible to zinc deficiency. Severe zinc deficiency, although uncommon, has been related to spontaneous abortion and congenital malformations (i.e., anencephaly), while milder forms have been linked with low birthweight, intrauterine growth retardation and preterm delivery.[17] Besides, mild zinc deficiency may also be related to complications of labor and delivery such as prolonged or inefficient first-stage and protracted second-stage labor, premature rupture of membranes, and the need for assisted or operative delivery.[17] The mechanisms underlying these associations are not clear, making it difficult to explain why such relationships have not been replicated in most randomized controlled trials (RCTs).
The Cochrane review on zinc supplementation in pregnancy by Mahomed et al. included 17 trials involving over 9000 women and their babies.[18] There was a significant, although small, reduction in preterm births based on 13 RCTs and 6854 women (RR: 0.86; 95% CI: 0.76-0.98) (Figure 2). The effect on low birthweight was nonsignificant (RR: 1.05; 95% CI: 0.94-1.17) based on 11 studies. Other primary maternal or neonatal outcomes such as pregnancy-induced hypertension or preeclampsia, prelabor rupture of membranes, antepartum hemorrhage, instrumental vaginal birth, maternal infection, postpartum hemorrhage, mean birthweight, small-for-gestational age, and infant morbidities such as neonatal sepsis, respiratory distress syndrome and neonatal intraventricular hemorrhage were no different between zinc and control groups. There was a small effect in favor of the zinc group for cesarean section (four trials with high heterogeneity) and for the induction of labor in a single trial.
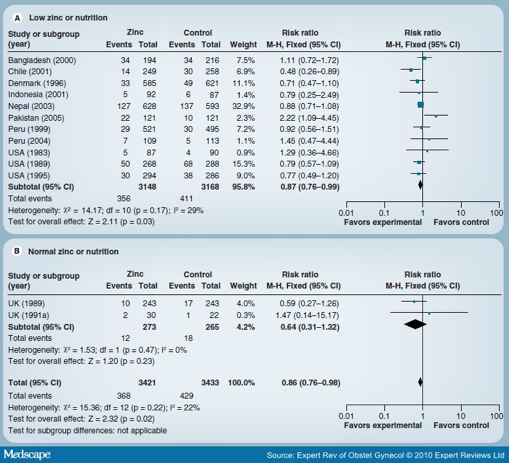
Figure 2. Forest plot of the effect of zinc versus no zinc on preterm birth. Data from [18].
Vitamin A
Vitamin A is an important micronutrient affecting the health of pregnant women and the fetus. Studies have shown that vitamin A deficiency is widespread throughout the developing world. Vitamin A deficiency has long been recognized in much of South and Southeast Asia (India, Bangladesh, Indonesia, Vietnam, Thailand and the Philippines) by the common presentation of clinical cases of xerophthalmia or night blindness, mostly in the latter half of the pregnancies.[19,20] Poor maternal vitamin A status affects its concentration in breast milk as well.[21,22] Vitamin A deficiency during pregnancy can result in fetal wastage, although high doses in early pregnancy can be teratogenic as well.[23] A cluster-randomized trial by West et al. from Nepal showed that all-cause maternal mortality up to 12 weeks postpartum was reduced by weekly vitamin A (RR: 0.60; 95% CI: 0.37-0.97) and β-carotene supplementation (RR: 0.51; 95% CI: 0.30-0.86) compared with the placebo group.[24] Fetal or early infant survival was not found to be improved with supplementation in this trial.[25] However, published data are awaited from other recent follow-up studies in Bangladesh (West KP et al. [2007], Unpublished Data) and Ghana (Kirkwood BR et al. [2009], Unpublished Data) that did not support such an effect of vitamin A on maternal mortality.
The potential role of vitamin A for the prevention of mother-to-child transmission (MTCT) of HIV has also been recently reviewed.[26] The review considered trials with HIV-infected pregnant women. There was no evidence of an impact of antenatal vitamin A supplementation on the risk of MTCT of HIV (three trials; 2022 women; RR: 1.05; 95% CI: 0.78-1.41) (Figure 3). It improved birthweight (MD: 89.78; 95% CI: 84.73-94.83), but with no impact on preterm birth, stillbirths or infant death. There was also no impact on maternal mortality based on one trial (RR: 0.49; 95% CI: 0.04-5.37).
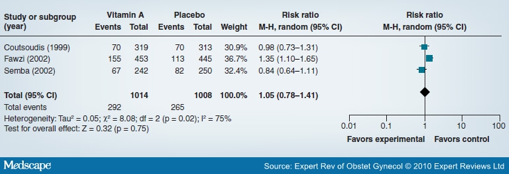
Figure 3. Forest plot of the impact of antenatal vitamin A supplementation in HIV-infected pregnant women on mother-to-child transmission of HIV. Data from [26].
Folic Acid
Pregnant and lactating women are at increased risk of folic acid deficiency because their dietary folic acid intake is insufficient to meet their physiological requirements and the metabolic demands of the growing fetus. The WHO compiled available information on the prevalence of anemia, which included the prevalence of folic acid deficiency. The percentage of pregnant women with a serum folic acid level of less than 3 ng/ml according to a WHO report in 1992 was highest among women in Sri Lanka (57%), followed by India (41.6%), Myanmar (13%) and Thailand (15%).[27,28] Maternal folic acid deficiency is associated with megaloblastic anemia owing to folic acid's role in DNA synthesis. Folic acid deficiency interferes with DNA synthesis, causing abnormal cell replication.[29] Low folic acid levels around the time of conception may cause neural tube defects (NTDs) in infants. Folic acid supplementation of women during the periconceptional period reduces the incidence of NTDs such as anencephaly and spina bifida.[30,31] The Cochrane review by Lumley et al. shows that periconceptional folic acid significantly reduces incidence of NTDs (RR: 0.28; 95% CI: 0.13-0.58) (Figure 4), including NTDs among women with (RR: 0.31; 95% CI: 0.14-0.66) and without (RR: 0.07; 95% CI: 0.00-1.33) prior NTDs.[32] Folate supplementation did not significantly increase rates of miscarriage, ectopic pregnancy or stillbirth, although there was a possible increase in multiple gestation. Increased risk of low-birthweight babies is also one of the major associations of low folic acid levels during pregnancy.[2] From a nutritional perspective, much of the interest in folic acid deficiency has centered around low birthweight and NTDs. However, with recent observations of elevated homocysteine levels in folic acid deficiency and the implications for increased risk of subsequent cardiovascular diseases, studies on folic acid deficiency should assume a higher priority.[33] For years folic acid has been supplemented with iron during pregnancy, mostly owing to its effects on the hematological system.[34] Its effects on other birth outcomes such as low birthweight, preterm delivery and perinatal mortality are still unclear.[35]
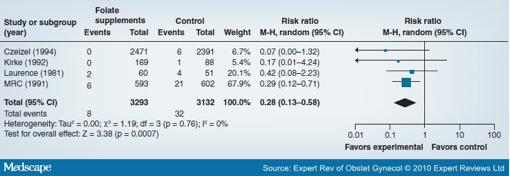
Figure 4. Forest plot of the impact of the use of periconceptional folate and/or multivitamins on neural tube defects. Data from [32].
Vitamin D
Maternal vitamin D deficiency is a widespread public health problem, especially in the developing world. Vitamin D deficiency during pregnancy has been linked with a number of serious short- and long-term health problems in offspring, including impaired growth, skeletal problems, Type 1 diabetes, asthma and schizophrenia.[36] Milk is only vitamin D-supplemented in a few countries, such as the USA. The major source of vitamin D is skin synthesis. With a high prevalence of vitamin D deficiency and poor dietary calcium intake, the problem is likely to worsen during pregnancy owing to the active transplacental transport of calcium to the developing fetus. Vitamin D deficiency early in pregnancy has been associated with a fivefold increased risk of preeclampsia.[37] Besides this, vitamin D deficiency during pregnancy has important consequences for the newborn, including fetal hypovitaminosis D, neonatal rickets and tetany, and infantile rickets.[38-40]
Vitamin D status at birth is closely related to that of the mother. The fetus at birth (cord blood) will contain approximately 50-60% of the maternal circulating concentrations of 25(OH)D.[41] Vitamin D supplementation during pregnancy may therefore help to improve the fetal and newborn vitamin D status and reduce the risk of vitamin D deficiency in the early months of life. The review by Mahomed et al. on vitamin D supplementation during pregnancy[42] reports data from two trials that had clinical outcomes.[43-46] In the London (UK) trial, the mothers had higher mean daily weight gain and a lower number of low-birthweight infants.[44-46] In the French trial, however, the supplemented group had lower birthweights.[43] There was an 87% reduction in the incidence of neonatal hypocalcemia (odds ratio [OR]: 0.13; 95% CI: 0.02-0.65), while no impact on craniotabes (softening of the skull) with supplementation (OR: 0.40; 95% CI: 0.09-1.65) was seen.
In the USA, the current dietary reference intake for vitamin D during pregnancy is 200-400 IU per day. However, there is data that supplementation of mothers with 400 IU per day during the last trimester of pregnancy did not significantly increase circulating 25(OH)D concentrations in the mothers or their infants at term.[47] Other studies have also shown that mothers who were deficient in vitamin D at the beginning of their pregnancy were still deficient at the end of their pregnancy, despite being supplemented with 800-1600 IU vitamin D per day throughout their pregnancy.[48] There are data to support the notion that doses exceeding 1000 IU vitamin D per day (2000-10,000 IU/day) are required to achieve a robust normal concentration of circulating 25(OH)D,[49-51] but this is an area that needs further research. Similarly, it is said that 400 IU of vitamin D per day during lactation is also inadequate, and insufficient to increase or even sustain the vitamin D status of mothers or their breastfeeding infants,[52] and that higher doses are recommended. More detailed studies are required to determine the appropriate vitamin D requirements of lactating mothers to achieve sufficient concentrations in breast milk.
Iodine
Iodine is required for the synthesis of thyroid hormones, which are required for the regulation of cell metabolism throughout the lifecycle.[102] Iodine deficiency is most serious in pregnant women and young children. During pregnancy, iodine deficiency adversely affects fetal development. Extreme iodine deficiency may cause fetal death (stillbirths or abortions), or severe physical and mental growth retardation, a condition known as cretinism.[104] Endemic cretinism is prevented by the correction of iodine deficiency, especially in women before and during pregnancy. The potential adverse effects of mild-to-moderate iodine deficiency during pregnancy are unclear. It can cause a range of problems in the fetus, referred to as iodine deficiency disorders: fetal loss, stillbirth, goiter, congenital anomalies and hearing impairment. The mental retardation resulting from iodine deficiency during pregnancy is irreversible.[105]
A review by Haider and Bhutta on iodine supplementation in pregnancy showed a 29% significant reduction in deaths during infancy and early childhood with maternal iodine supplementation (two studies; RR: 0.71; 95% CI: 0.56-0.90) (Figure 5).[53] Similarly, there was a 73% reduced risk of endemic cretinism at 4 years of age with iodine supplementation in pregnancy (one study; RR: 0.27; 95% CI: 0.12-0.60). This shows the public health importance of iodine supplementation in pregnancy to avoid adverse outcomes in the fetus or child, especially in areas where iodine deficiency is endemic.
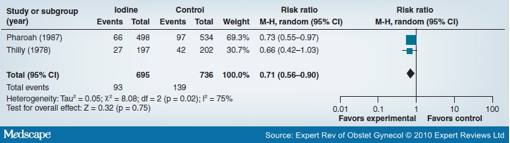
Figure 5. Forest plot of the impact of iodine supplementation versus no iodine in pregnancy on infant and early childhood mortality. Data from [53].
Calcium
Calcium is required for the skeletal development of the fetus.[7] Calcium also plays a role in neuromuscular function and blood coagulation. Besides the effect of calcium on bone and mineral development of the fetus, its deficiency alters membrane permeability and smooth muscle contractility, which in turn could affect blood pressure, as well as lead to premature uterine contractions and subsequent delivery.[7]
Calcium supplementation during pregnancy reduces the risk of high blood pressure (with or without proteinuria) by 30% (RR: 0.70; 95% CI: 0.57-0.86).[54] It also significantly reduced the risk of preeclampsia by 52% (RR: 0.48; 95% CI: 0.33-0.69), but significance was achieved only in women on a low-calcium diet for this outcome. Maternal death/serious morbidity was also reduced (RR: 0.80; 95% CI: 0.65-0.97).[54] There was no overall effect on preterm birth (ten trials; 14,751 women; RR: 0.81; 95% CI: 0.64-1.03). According to a recent article, calcium intake was not a significant predictor of skeletal response to pregnancy in well-nourished women.[55] At present, there is also no strong evidence demonstrating that improving maternal calcium status has a long-term positive effect on childhood bone mass.[56]
Magnesium
Dietary intake studies during pregnancy have consistently shown below-recommended intakes of magnesium, especially in those from disadvantaged backgrounds.[57] Preeclampsia was not shown to be affected by magnesium supplementation. Many studies from developed countries, however, have found fewer preterm births and less intrauterine growth retardation with magnesium supplementation during pregnancy.[58-60] Unfortunately, data are not available from developing countries. One of the recent reviews of micronutrient efficacy concluded that the only supplements that affected birthweight were magnesium (which reduced small-for-gestational age births by 30%) and calcium (which reduced the risk of low birthweight).[61] The review by Makrides et al. on magnesium supplementation included seven trials, recruiting 2689 women.[62] Magnesium treatment was associated with a lower frequency of preterm births (RR: 0.73; 95% CI: 0.57-0.94) compared with placebo. However, there was no impact on the risk of preeclampsia, miscarriage, stillbirth or neonatal mortality. Maternal hospitalization was significantly reduced with magnesium treatment, and a reduction was also seen in antepartum hemorrhage. The risk of low birthweight and small-for-gestational age babies was also significantly reduced.
Other Vitamins and Minerals
There have been associations of thiamine, vitamin B6 and B12 deficiencies with fetal development,[7] but they remain largely of unknown importance for pregnancy outcomes. In a controlled trial in HIV-infected women, Fawzi et al. found significant reductions in intrauterine growth retardation and preterm births, as well as lower perinatal mortality with high-dose B vitamins, as well as vitamins C and E.[63] Interest has also been increasing with the observation that homocysteinemia is associated with adverse pregnancy outcomes. Deficiencies in vitamin B (riboflavin, B6 and B12) are known to increase the level of homocysteine in plasma, which in turn has been shown to be associated with placental abruption, stillbirth, very low birthweight and preterm deliveries, as well as higher rates of preeclampsia and NTDs in the offspring.[64] Apart from studies on folate, there are few data on the relationship between the B-vitamin status of pregnant women and risk of adverse pregnancy outcomes. The early prevention of B-vitamin deficiencies is also important to prevent the iron depletion throughout pregnancy, and to prevent preterm delivery.[65]
The review by Rumbold et al. on vitamin C supplementation in pregnancy included five trials involving 766 women.[66] The risk of stillbirth (three trials; 539 women; RR: 0.87; 95% CI: 0.41-1.87), perinatal death (two trials; 238 women; RR: 1.16; 95% CI: 0.61-2.18), birthweight (one trial; 100 women; weighted MD: -139.00 g; 95% CI: -517.68-239.68) or intrauterine growth restriction (two trials; 383 women; RR: 0.72; 95% CI: 0.49-1.04) was not different between women supplemented with vitamin C alone or in combination with other supplements and placebo. Women supplemented with vitamin C compared with placebo were at increased risk of giving birth preterm (three trials; 583 women; RR: 1.38; 95% CI: 1.04-1.82). The risk of preeclampsia was significantly decreased in the fixed-effect model (four trials; 710 women; RR: 0.47; 95% CI: 0.30-0.75); however, this difference could not be demonstrated when using a random-effects model (four trials; 710 women; RR: 0.52; 95% CI: 0.23-1.20). The vitamin E review had four trials on 566 women at high risk or with preeclampsia, and showed results similar to those of vitamin C supplementation.[67] There was no difference in the risk of stillbirth, neonatal death, perinatal death, preterm birth, intrauterine growth restriction or birthweight. The risk of clinical preeclampsia was decreased in the fixed-effect models (three trials; 510 women; RR: 0.44; 95% CI: 0.27-0.71).
Regarding selenium supplementation, a randomized trial from Tanzania on HIV-infected pregnant women showed no significant effect on maternal CD4 cell counts or viral load.[10] There was a marginal impact on low birthweight (RR: 0.71; 95% CI: 0.49-1.05) and fetal death (RR: 1.58; 95% CI: 0.95-2.63). It also had no effect on maternal, neonatal or overall child mortality, but reduced the risk of child mortality after 6 weeks (RR: 0.43; 95% CI: 0.19-0.99). Studies have shown that selenium and copper deficiencies/excesses may also be associated with adverse outcomes of pregnancy. In a cross-sectional study of 166 Zairian pregnant women, low birthweight infants had a mean umbilical serum selenium concentration lower than normal birthweight infants (p < 0.02).[68] In another observational study, serum selenium concentrations were significantly lower in preterm infants than full-term infants (0.54 vs 0.60 mumol/l; p = 0.01), whereas infants that were small-for-gestational age did not differ in serum selenium concentrations from infants that were adequate-for-gestational age. Serum selenium concentrations in malformed infants tended to be lower than in normal infants (0.55 vs 0.60 mumol/l; p = 0.09), but the difference was not statistically significant, probably owing to the small number.[69] Among HIV-infected women, low selenium status may increase the risk of MTCT of HIV and poor pregnancy outcomes such as low birthweight, small-for-gestational age, preterm birth and fetal death.[70] High zinc and copper levels have been associated with preterm birth and low birthweight. Excess zinc can cause a suppressed immune response, decreased high-density lipoprotein cholesterol and a reduced copper status.[71] In an observational study in Poland, Wasowicz et al. found that both zinc and copper concentrations were significantly higher in plasma of preterm infants compared with full-term infants.[72] The mothers of preterm infants and low birthweight babies also had significantly higher plasma copper concentrations. Similarly, serum copper measured at delivery was negatively associated with birthweight in another cross-sectional study.[73] These findings warrant better-designed prospective studies.
Multiple Micronutrient Supplements
Although micronutrient deficiencies exist in pregnant women, supplementation is provided for only one or two micronutrients, with observed benefits on child growth, health, neurodevelopment and pregnancy outcomes. As indicated previously, many population groups in the developing world suffer from multiple nutrient deficiencies. Concurrent deficiencies of vitamin A, zinc, iron and iodine have been recognized in women of reproductive age and during pregnancy. Given that mere changes in dietary habits are considered insufficient to meet micronutrient requirements in pregnancy and alleviate pre-existing deficits, in 1999 the UNICEF/WHO/UN University proposed a prenatal supplement known as the United Nations International Multiple Micronutrient Preparation (UNIMMAP) containing 15 micronutrients, including iron and folic acid, which could provide one recommended daily allowance of each.[74] It consists of iron 30 mg, folic acid 400 µg, zinc 15 mg, copper 2.0 mg, selenium 65 µg, vitamin A 800 µg retinol equivalent, vitamin B1 1.4 mg, vitamin B2 1.4 mg, niacin 18 mg, vitamin B6 1.9 mg, vitamin B12 2.6 µg, vitamin C 70 mg, vitamin D 5 µg, vitamin E 10 mg and iodine 150 µg. It is hoped that these multiple-micronutrient supplements (MMSs) could potentially replace standard iron-folate supplements for pregnant women in low- and middle-income countries. The micronutrients given in such a combination can possibly have additive or even synergistic effects on the health of the mother and her baby, and be a cost-effective strategy to address multiple-micronutrient deficiencies in developing countries. It should, however, be noted that in RCTs on UNIMMAP, the control group was given 60 mg iron, while the UNIMMAP contained only 30 mg iron. Thus, some of the benefit of UNIMMAP may have been reduced if iron dosages higher than 30 mg are considered to be necessary.
The latest review on MMSs in pregnancy is the series of three papers in the Food and Nutrition Bulletin.[75-77] Multiple micronutrients compared with controls (receiving iron-folate) increased the mean birthweight (pooled estimate: +22.4 g; 95% CI: 8.3-36.4 g) and significantly reduced the incidence of low birthweight (pooled OR: 0.89; 95% CI: 0.81-0.97) and small-for-gestational age (pooled OR: 0.90; 95% CI: 0.82-0.99). In mothers with a BMI of 20 kg/m2 or higher, the intervention effect on birthweight remained significant: +39.0 g (95% CI: +22.0 to +56.1 g), compared with -6.0g (95% CI: -28.8 to +16.8 g) in mothers with a BMI of less than 20 kg/m2. The difference was highly significant (p < 0.001) and did not change after adjustment for maternal age and education. Multiple micronutrients were not associated with an increase in gestation or reduction in preterm births. Furthermore, MMS was not associated with the incidence of stillbirth (OR: 1.01; 95% CI: 0.88-1.16), but there was a nonsignificant 23% increase in early neonatal mortality (OR: 1.23; 95% CI: 0.96-1.59) and a nonsignificant 11% increase in perinatal mortality (OR: 1.11; 95% CI: 0.93-1.33). The pooled data also showed a nonsignificant 6% reduction in late neonatal mortality (OR: 0.94; 95% CI: 0.73-1.23). The aforementioned series did not cover any maternal outcomes. In the review by Haider and Bhutta, supplementation with multiple micronutrients versus iron-folate was associated with comparable effects on maternal anemia based on one trial (RR: 1.23; 95% CI: 0.82-1.83).[78] A randomized trial on multivitamin supplements (vitamin B complex, vitamin C and vitamin E) found that a single dose of the recommended dietary allowance (RDA) may be as efficacious as multiple doses of the RDA in decreasing the risk of adverse pregnancy outcomes among HIV-infected women,[79] and the effects of multiple and single doses were similar on low birthweight, preterm birth, small-for-gestational age, and fetal or early infant death.
There is considerable debate on the efficacy of providing multiple micronutrient supplements during pregnancy and concerns have been expressed on potential adverse effects with an increase in neonatal mortality in less developed healthcare systems with suboptimal maternal care.[1,80] This may not be relevant in circumstances where skilled care and facility births are available. Further studies are needed to determine the effectiveness and safety of MMS in health system settings.
Suggested Interventional Strategies
Maternal nutrition interventions to improve maternal and neonatal health include supplementation with micronutrients such as iron, folate and calcium, and also macronutrients such as balanced energy and protein diets. The review by Bhutta et al. on interventions to address maternal and child undernutrition derived the data of over 388 studies from 139 countries to find preventive and therapeutic strategies to address undernutrition deficiencies.[53] These interventions include disease-control measures, as well as dietary diversification, supplementation and food-fortification strategies. Maternal nutrition interventions are not only entirely feasible, but also affordable and cost effective. Nutrition interventions are among the best investments in development that countries can undertake, provided that these can be implemented at scale with community participation. Large-scale programs, including the provision of vitamins and minerals through fortified foods and supplements during pregnancy, have been successful in many countries. At the Copenhagen Consensus in 2008, micronutrient supplementation and fortification were identified as among the most cost-effective interventions to address infant and child undernutrition globally, with a cost-benefit ratio of 9.5-17.3 (estimated annual gains of US$3.7 billion for net investments of US$346.4 million). However, other than iron fortification, the group identified no strategies for addressing the size of the problem among women of reproductive age.[81]
Supplementation Strategies
The usual approach to improving micronutrient status during pregnancy - that is, for iron and folic acid - is supplementation. This approach has been beset with many problems, including limited supplies, lack of trained healthcare workers, and poor compliance to the supplementation schedule either owing to side effects or other reasons, with suboptimal effects on anemia.[82] More research is needed on the routine use of MMS during pregnancy in developing country settings before it can replace the decades-old iron-folic acid, especially in settings where facility-based deliveries are not available and overall maternal care is suboptimal. In addition, attention should be focused on other complementary strategies, such as the fortification of foods with micronutrients, which may prove to be more sustainable and do away with the problem of compliance.
Fortification
This may be an alternative strategy to provide essential micronutrients through the fortification of staple foods or other products. Iron fortification increases the hemoglobin level in women of child-bearing age and during pregnancy.[83] Studies have shown larger effects on hemoglobin by fortified food in comparison to other interventions. Along with the iodization of salt, adding such vitamins and minerals such as iron, zinc, vitamin A and folic acid to staple foods and condiments is a cost-effective way to improve the vitamin and mineral intake of the overall population, including women of reproductive age. As of March 2009, approximately 30% of the world's wheat flour produced in large roller mills was fortified, while 57 countries had legislation mandating the fortification of one or more types of flour with either iron or folic acid.[106] Although many foods such as fats, oils and margarine have been fortified for years in some countries, this approach has not yet been scaled up in many lower income countries.[84] Through increased efforts by various partnerships and alliances, it is expected that food fortification will continue to gain momentum.
There are, however, many challenges to the food fortification programs and several factors must be taken into consideration. The carrier needs to be a staple food of the target population. In addition, centralized processing is necessary, and frequent as well as reasonably constant consumption is desirable. The fortifying agent must have adequate physicochemical, organoleptic and bioavailability characteristics. This means that the color, taste, odor and appearance of the carrier food must not be affected. The total cost of the final product must not significantly increase with fortification. Besides, it is necessary to have a monitoring and control system that guarantees both adequate nutrient concentration and program compliance. In case of potentially toxic nutrients, excessive concentrations should not be added that could put a population at risk. Legal issues include the facts of whether the program should be compulsory or voluntary, and whether it should be financed by the government or the private sector, or both. Even if food fortification programs are mandatory, they may fail to secure compliance; therefore, different approaches for adequate implementation may be adopted, possibly including fiscal and tariff incentives.[107] It is also important to demonstrate and document the cost-benefit ratio of the intervention in order to gain objective evidence to support the continuance of these programs.[85] Besides, in areas where subsistence farming is practiced, fortification is not likely to be a practicable solution to nutrient deficiencies.
Technologically, it would be necessary to maintain the overall quality of the product in terms of the bioavailability of the fortifying agent. Despite an increase in bioavailability, the product's quality may be at risk, especially its stability. Iron, for example, may react with fatty acids in the fortified food, forming free radicals that induce oxidation. Any alterations that affect consumer acceptability of the product should be avoided. Some options for the future include microencapsulation of nutrients, the use of nutrient-bioavailability stimulants (the addition of ascorbic or other organic acids to promote iron absorption) and the elimination of inhibitors of mineral absorption in the intestine (e.g., phytates).[107] Fortification is certainly an effective micronutrient deficiency control strategy whose coverage needs to be expanded. It should, however, be viewed as a complementary strategy, and it will not be able to replace interventions such as iron supplementation during pregnancy (and other micronutrient control strategies among other population groups), at least in the short term.
Dosage and Potential Interactions
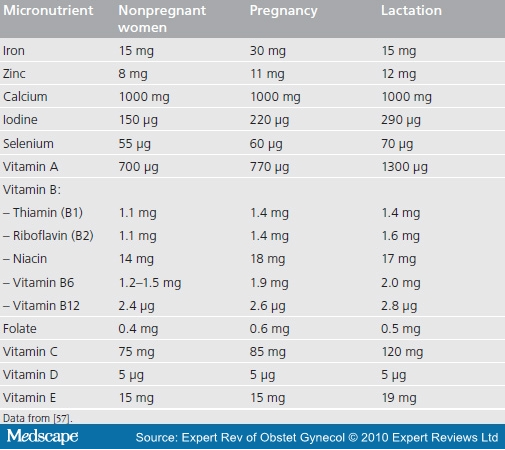
Table 1 shows the recommended daily micronutrient intake during pregnancy and lactation based on recommendations provided by the American Academy of Pediatrics and American College of Obstetricians and Gynecologists.[57] The current recommendation for pregnant women is to provide a standard daily dose of 60 mg iron and 400 µg folic acid for 6 months, or if this duration of treatment cannot be achieved during pregnancy, then either to continue supplementation during the postpartum period or to increase the dose of iron to 120 mg daily during pregnancy.[86] In those areas where iron is fortified in foods, the recommended daily dose of iron during pregnancy is 30 mg. Given that multiple deficiencies can coexist, we need to determine the efficacy of multiple micronutrients to replace iron-folate for pregnant women in developing countries, even though their use has become common practice in developed countries.[87]
Table 1. Recommended daily micronutrient intake during pregnancy and lactation.
Interactions and Potential Adverse Effects
Constipation is a common side effect of high-dose iron supplements, with other gastrointestinal effects being nausea, vomiting and diarrhea.[14] The frequency and severity of these side effects varies according to the amount of elemental iron released in the stomach, and is one of the main reasons for poor compliance with iron preparations in pregnancy. Besides this, there is growing evidence of the existence of metabolic interactions between micronutrients such as copper, zinc and iron. Excess iron can lower zinc nutritional status and vice versa;[88,89] similarly, copper and zinc compete with each other.[90,91] Vitamin A deficiency contributes to anemia by interfering with iron utilization.[87] Besides vitamin A, other micronutrients can also enhance the absorption of other micronutrients, such as vitamin C, increasing iron bioavailability.[92] As can be seen, these interactions may be positive or negative, and we do not have significant scientific data on this aspect. The issues related to the interactions between micronutrients and the coexistence of micro- and macro-nutrient deficiencies require serious considerations before MMSs are given to pregnant women.
Conclusion
Micronutrient deficiencies during pregnancy, either singly or in combination, can have important effects on pregnancy outcomes. Interventions; for example, folic acid supplementation, should start well before conception (in the reproductive age) to prevent adverse effects such as NTDs, as embryogenesis occurs early in pregnancy and the woman must have adequate micronutrient levels to offset any deficiency before and early in pregnancy. For other outcomes, interventions after the pregnancy is detected may be effective. Iron and folic acid supplementation is currently recommended for all pregnant women in developing countries. In population groups where the prevalence of anemia is above 20% among women of reproductive age and mass fortification programs of staple foods with iron and folic acid are unlikely to be implemented within 1-2 years, weekly iron-folic acid supplementation should be considered as a strategy for the prevention of iron deficiency. The weekly supplement should contain 60 mg iron in the form of ferrous sulfate (FeSO4.7H2O) and 2800 µg folic acid.[108]
Multiple micronutrient supplements are routinely used by pregnant women in developed nations and by the rich in developing countries, but these are not currently routinely prescribed to pregnant women in low- or middle-income countries in lieu of iron-folate. The increase in birthweight and reduction in small-for-gestational age births by MMS means that such interventions need to be complemented with facility-based care for delivery (intrapartum care) to preempt and prevent potentially adverse neonatal outcomes due to obstructed labor and possible birth asphyxia. MMSs are also known to be more effective in women with a higher BMI;[75] therefore, food supplements may also be considered in addition to MMSs to overcome wasting, which may also worsen pregnancy outcomes. Apart from supplementation, other complementary dietary approaches should also be considered, such as fortification. Fortification can effectively address prepregnancy folate deficiency and help improve iron status among women of reproductive age. It can provide improved micrountrient intake and help improve maternal nutrition, as well as pregnancy outcomes. However, fortification cannot obviate the need for iron (and folate) supplementation during pregnancy in most settings.
Expert Commentary
Existing and emerging research linking micronutrient deficiencies during pregnancy with adverse birth outcomes is an exciting development. Supplementation of micronutrients; for example, iron-folate, has been the decades-old way of preventing and treating iron-deficiency anemia during pregnancy. In those areas where the prevalence of anemia is high and mass fortification of iron is unlikely to meet the needs of the population, weekly rather than daily iron supplementation is recommended for women of the reproductive age group. Folic acid supplementation has a definite role to play in reducing both occurrent (first time) and recurrent NTDs. Micronutrient supplementation can also have a positive impact on other birth outcomes: zinc and magnesium supplementation reduces preterm births, the effects being small but significant. Their recommendation for pregnancy, however, is still weak. For many micronutrient deficiencies such as zinc, the mechanism by which they are associated with adverse pregnancy outcomes is unclear, and such associations have not been replicated in most RCTs. For some micronutrients; for example, copper, RCTs of its supplementation during pregnancy are lacking. This is an area of future research.
Deficiencies of multiple micronutrients may coexist, suggesting supplementation in one tablet with more than one micronutrient, including iron-folate. The efficacy of the UNIMMAP supplement is still being determined, and multiple micronutrients, although widely used in developed countries and among the wealthy in developing countries, are still not routinely used for pregnant women in developing countries. The composition of iron in UNIMMAP is 30 mg, which may be less than the recommended dose of 60 mg daily iron by the WHO. Further research in terms of more RCTs needs to be carried out to establish their efficacy for women in the developing world. There is some concern about a possible increase in early neonatal mortality with the MMS supplements, and so more research needs to be conducted in this regard.
Five-year View
Micronutrient supplementation during pregnancy is an emerging field. In the next 5 years, more work is expected, especially on formulations such as UNIMMAP. The coming years will establish whether MMSs are feasible for prescription to pregnant women in lieu of iron-folate. More RCTs will come into play that will establish if they have an adverse impact on neonatal mortality, especially in areas where intrapartum maternal care is suboptimal. The importance of food supplements to prevent or overcome wasting with MMS will also come into light, as MMSs are found to be more effective in women with a higher BMI.
Relating to single micronutrients, research with regards to the mechanism behind how zinc deficiency causes congenital anomalies and if zinc is also important periconceptionally such as folic acid to prevent congenital malformations will arise. Future work will also focus on discovering the molecular mechanisms by which deficiencies of different micronutrients impact on pregnancy outcomes; for example, the association of zinc with the premature rupture of membranes and prolonged labor. More research is also expected with regards to how folic acid deficiency causes NTDs and on the relationship of folic acid deficiency with low birthweight. Other interesting areas awaiting further research include the impact of vitamin A supplementation on maternal mortality, as recent trials from Ghana and Bangladesh have shown no effect of vitamin A supplementation on this outcome. The future may also see more RCTs on the supplementation of micronutrients such as copper and selenium, considered less important at the moment, and whether this helps to reduce adverse pregnancy outcomes or not. Copper supplementation during pregnancy is one field that is a major research gap at present, as little is known whether it is of benefit or detriment in terms of adverse pregnancy outcomes, as current data are only available based on observational studies.
Key Issues
• Nutritional deficiencies are widely prevalent globally and contribute significantly to maternal and child morbidity and mortality.
• Micronutrient deficiencies are associated with adverse pregnancy outcomes such as low birthweight and preterm birth.
• Iron supplementation reduces the risk of anemia in mothers at term.
• Folic acid supplementation reduces the incidence of neural tube defects.
• Zinc supplementation has a small but significant impact on preterm births; however, its recommendation is weak.
• Vitamin A may reduce maternal mortality, but results of ongoing studies are awaited.
Other minerals and vitamins, such as iodine, calcium, magnesium, vitamin
D, selenium, and vitamins B6, B12, C and E, may also be important for maternal,
infant and child outcomes.
References
1. Bhutta ZA, Haider BA. Prenatal micronutrient supplementation: are we there yet CMAJ180(12),1188-1189 (2009).
2. Seshadri S. Prevalence of micronutrient deficiency particularly of iron, zinc and folic acid in pregnant women in South East Asia. Br. J. Nutr.85(Suppl. 2),S87-S92 (2001).
3. Bell AW, Ehrhardt RA. Regulation of placental nutrient transport and implications for fetal growth. Nutr. Res. Rev.15(2),211-230 (2002).
4. Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev. Reprod.2(2),105-112 (1997).
5. Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J. Nutr.134(9),2169-2172 (2004).
6. Black RE, Allen LH, Bhutta ZA et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet371(9608),243-260 (2008).
7. Ramakrishnan U, Manjrekar R, Rivera J, Gonzales-Cossio T, Martorell R. Micronutrients and pregnancy outcome: a review of the literature. Nutr. Res.19,103-159 (1999).
8. de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull.29(2 Suppl.),S238-S244 (2008).
9. Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am. J. Clin. Nutr.88(2),520S-528S (2008).
10. Kupka R, Mugusi F, Aboud S et al. Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: effects on maternal and child outcomes. Am. J. Clin. Nutr.87(6),1802-1808 (2008).
11. Indian Council of Medical Research. Task Force Study. Evaluation of National Nutritional Anemia Prophylaxis Programme. Indian Council of Medical Research, New Delhi, India (1989).
12. Rasmussen K. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality J. Nutr.131(2S-2),590S-601S; discussion 601S-603S (2001).
13. Ezzati M, Lopez AD, Rodgers A, Murray CJL. Comparative quantification of health risks. Global and regional burden of disease attributable to selected major risk factors. WHO, Geneva, Switzerland (2004).
14. Pena-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database Syst. Rev.4,CD004736 (2009).
14.•• Comprehensive review of iron supplementation during pregnancy, with different outcomes reported and the latest recommendations on iron supplementation.
15. Christian P, Stewart CP, LeClerq SC et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow-up in a randomized, controlled community trial. Am. J. Epidemiol.170(9),1127-1136 (2009).
15.• Unique article showing the impact of antenatal and postnatal iron supplementation on childhood mortality.
16. Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am. J. Clin. Nutr.68(2 Suppl.),499S-508S (1998).
17. Jameson S. Zinc status in pregnancy: the effect of zinc therapy on perinatal mortality, prematurity, and placental ablation. Ann. NY Acad. Sci.678,178-192 (1993).
18. Mahomed K, Bhutta Z, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev.2,CD000230 (2007).
19. Katz J, Khatry SK, West KP et al. Night blindness is prevalent during pregnancy and lactation in rural Nepal. J. Nutr.125(8),2122-2127 (1995).
20. Christian P, West KP Jr, Khatry SK et al. Night blindness of pregnancy in rural Nepal - nutritional and health risks. Int. J. Epidemiol.27(2),231-237 (1998).
21. Ross JS, Harvey PW. Contribution of breastfeeding to vitamin A nutrition of infants: a simulation model. Bull. World Health Organ.81(2),80-86 (2003).
22. Lonnerdal B. Effects of maternal dietary intake on human milk composition. J. Nutr.116(4),499-513 (1986).
23. Azais-Braesco V, Pascal G. Vitamin A in pregnancy: requirements and safety limits. Am. J. Clin. Nutr.71(5 Suppl.),1325S-1333S (2000).
24. West KP Jr, Katz J, Khatry SK et al.; The NNIPS-2 Study Group. Double blind, cluster randomised trial of low dose supplementation with vitamin A or b carotene on mortality related to pregnancy in Nepal. Br. Med. J.318(7183),570-575 (1999).
25. Katz J, West KP Jr, Khatry SK et al. Maternal low-dose vitamin A or ß-carotene supplementation has no effect on fetal loss and early infant mortality: a randomized cluster trial in Nepal. Am. J. Clin. Nutr.71(6),1570-1576 (2000).
26. Wiysonge CS, Shey MS, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst. Rev.4,CD003648 (2005).
27. WHO. The prevalence of anemia in women: a tabulation of available information. WHO, Geneva, Switzerland (1992).
28. Basu RN, Sood SK, Ramachandran K, Mathur M, Ramalingaswami V. Etiopathogenesis of nutritional anemia in pregnancy: a therapeutic approach. Am. J. Clin. Nutr.26(6),591-594 (1973).
29. Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br. Med. Bull.55(3),578-592 (1999).
30. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med.150(9),626-631 (2009).
31. No authors listed. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet338(8760),131-137 (1991).
32. Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst. Rev.3,CD001056 (2001).
32.• Comprehensive review of folate supplementation during pregnancy that shows a strong significant impact on the reduction of neural tube defects.
33. Johnston RB. Folic acid: preventive nutrition for preconception, the fetus, and the newborn. Neoreviews10,e10-e19 (2009).
34. Fleming AF, Ghatoura GB, Harrison KA, Briggs ND, Dunn DT. The prevention of anaemia in pregnancy in primigravidae in the guinea savanna of Nigeria. Ann. Trop. Med. Parasitol.80(2),211-233 (1986).
35. Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am. J. Clin. Nutr.71(5 Suppl.),1295S-1303S (2000).
36. Holick MF. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest.116(8),2062-2072 (2006).
37. Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab.92(9),3517-3522 (2007).
38. Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. J. Pediatr.109(2),328-334 (1986).
39. Purvis RJ, Barrie WJ, MacKay GS, Wilkinson EM, Cockburn F, Belton NR. Enamel hypoplasia of the teeth associated with neonatal tetany: a manifestation of maternal vitamin-D deficiency. Lancet2(7833),811-814 (1973).
40. Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am. J. Clin. Nutr.81(5),1060-1064 (2005).
41. Hollis BW, Pittard WB 3rd. Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J. Clin. Endocrinol. Metab.59(4),652-657 (1984).
42. Mahomed K, Gulmezoglu AM. Vitamin D supplementation in pregnancy. Cochrane Database Syst. Rev.2,CD000228 (2000).
43. Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet. Gynecol.68(3),300-304 (1986).
44. Brooke OG, Brown IR, Bone CD et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br. Med. J.280(6216),751-754 (1980).
45. Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br. Med. J. (Clin. Res. Ed.)283(6298),1024 (1981).
46. Maxwell JD, Ang L, Brooke OG, Brown IR. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. Br. J. Obstet. Gynaecol.88(10),987-991 (1981).
47. Cockburn F, Belton NR, Purvis RJ et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. Br. Med. J.281(6232),11-14 (1980).
48. Datta S, Alfaham M, Davies DP et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population - an interventional study. BJOG109(8),905-908 (2002).
49. Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr.73(2),288-294 (2001).
50. Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr.80(6 Suppl.),1752S-1758S (2004).
51. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr.77(1),204-210 (2003).
52. Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am. J. Clin. Nutr.79(5),717-726 (2004).
53. Bhutta ZA, Ahmed T, Black RE et al. What works Interventions for maternal and child undernutrition and survival. Lancet371(9610),417-440 (2008).
53.•• Comprehensive review of interventions of different micronutrients to reduce the burden of maternal and child undernutrition, including a series of systematic reviews on micronutrients such as iodine in the web appendices.
54. Hofmeyr GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev.3,CD001059 (2006).
55. Olausson H, Laskey MA, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. Am. J. Clin. Nutr.88(4),1032-1039 (2008).
56. Abrams SA. In utero physiology: role in nutrient delivery and fetal development for calcium, phosphorus, and vitamin D. Am. J. Clin. Nutr.85(2),604S-607S (2007).
57. Subcommittee on Nutritional Status and Weight Gain During Pregnancy, Subcommittee on Dietary Intake and Nutritent Supplements During Pregnancy, Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences. Nutrition During Pregnancy. National Academy Press, Washington DC, USA (1990).
58. Conradt A, Weidinger H, Algayer H. Magnesium therapy decreased the rate of intrauterine fetal retardation, premature rupture of membranes and premature delivery in risk pregnancies treated with betamimetics. Magnesium4(1),20-28 (1985).
59. Spatling L, Spatling G. Magnesium supplementation in pregnancy. A double-blind study. Br. J. Obstet. Gynaecol.95(2),120-125 (1988).
60. Sibai BM, Villar MA, Bray E. Magnesium supplementation during pregnancy: a double-blind randomized controlled clinical trial. Am. J. Obstet. Gynecol.161(1),115-119 (1989).
61. Merialdi M, Carroli G, Villar J et al. Nutritional interventions during pregnancy for the prevention or treatment of impaired fetal growth: an overview of randomized controlled trials. J. Nutr.133(5 Suppl. 2),1626S-1631S (2003).
62. Makrides M, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev.4,CD000937 (2001).
63. Fawzi WW, Msamanga GI, Spiegelman D et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet351(9114),1477-1482 (1998).
64. Vollset SE, Refsum H, Irgens LM et al. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am. J. Clin. Nutr.71(4),962-968 (2000).
65. Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. Am. J. Clin. Nutr.81(5),1206S-1212S (2005).
66. Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst. Rev.2,CD004072 (2005).
67. Rumbold A, Crowther CA. Vitamin E supplementation in pregnancy. Cochrane Database Syst. Rev.2,CD004069 (2005).
68. Arnaud J, Preziosi P, Mashako L et al. Serum trace elements in Zairian mothers and their newborns. Eur. J. Clin. Nutr.48(5),341-348 (1994).
69. Bro S, Berendtsen H, Norgaard J, Host A, Jorgensen PJ. Serum selenium concentration in maternal and umbilical cord blood. Relation to course and outcome of pregnancy. J. Trace Elem. Electrolytes Health Dis.2(3),165-169 (1988).
70. Kupka R, Garland M, Msamanga G, Spiegelman D, Hunter D, Fawzi W. Selenium status, pregnancy outcomes, and mother-to-child transmission of HIV-1. J. Acquir. Immune Defic. Syndr.39(2),203-210 (2005).
71. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. National Academy Press, Washington DC, USA (2001).
72. Wasowicz W, Wolkanin P, Bednarski M, Gromadzinska J, Sklodowska M, Grzybowska K. Plasma trace element (Se, Zn, Cu) concentrations in maternal and umbilical cord blood in Poland. Relation with birth weight, gestational age, and parity. Biol. Trace Elem. Res.38(2),205-215 (1993).
73. Ghebremeskel K, Burns L, Burden TJ et al. Vitamin A and related essential nutrients in cord blood: relationships with anthropometric measurements at birth. Early Hum. Dev.39(3),177-188 (1994).
74. UNICEF/WHO/UNU. Composition of a multi-micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. UNICEF, NY, USA (1999).
75. Fall CHD, Fisher DJ, Osmond C, Margetts BM; the Maternal Micronutrient Supplementation Study Group (MMSSG). Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr. Bull.30(4),S533-S546 (2009).
75.•• Very useful latest paper of the impact of multiple-micronutrient supplements MMS on birthweight and length of gestation. Also shows that MMS are more effective in women with a higher BMI.
76. Margetts BM, Fall CHD, Ronsmans C, Allen LH, Fisher DJ; Maternal Micronutrient Supplementation Study Group (MMSSG). Multiple micronutrient supplementation during pregnancy in low-income countries: review of methods and characteristics of studies included in the meta-analyses. Food Nutr. Bull.30(4),S517-S526 (2009).
77. Ronsmans C, Fisher DJ, Osmond C, Margetts BM, Fall CHD; Maternal Micronutrient Supplementation Study Group (MMSSG). Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on stillbirths and on early and late neonatal mortality. Food Nutr. Bull.30(4),S547-S55 5 (2009).
78. Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev.4,CD004905 (2006).
79. Kawai K, Kupka R, Mugusi F et al. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV-infected women in Tanzania. Am. J. Clin. Nutr.91(2),391-397 (2009).
80. Bhutta ZA, Haider BA. Maternal micronutrient deficiencies in developing countries. Lancet371(9608),186-187 (2008).
81. Horton S, Alderman H, Rivera JA. Copenhagen Consensus 2008 Challenge Paper: Hunger and Malnutrition. Copenhagen Consensus Center, Copenhagen, Denmark (2008).
82. ACC/SCN. Second Report on the World Nutrition Situation. Global and Regional Results (Volume 1). ACC/SCN, Geneva, Switzerland (1992).
83. Van Thuy P, Berger J, Nakanishi Y, Khan NC, Lynch S, Dixon P. The use of NaFeEDTA-fortified fish sauce is an effective tool for controlling iron deficiency in women of childbearing age in rural Vietnam. J. Nutr.135(11),2596-2601 (2005).
84. Darnton-Hill I. Overview: rationale and elements of a successful food-fortification programme. Food Nutr. Bull.19(2),92-100 (1998).
85. Chopra JG. Enrichment and fortification of foods in Latin America. Am. J. Public Health64(1),19-26 (1974).
86. WHO. Iron and Folate Supplementation. Standards for Maternal and Neonatal Care. Integrated Management of Pregnancy and Childbirth (IMPAC). Department of Making Pregnancy Safer (MPS), WHO, Geneva, Switzerland (2006).
87. Black RE. Micronutrients in pregnancy. Br. J. Nutr.85(Suppl. 2),S193-S197 (2001).
88. Hambidge KM, Krebs NF, Sibley L, English J. Acute effects of iron therapy on zinc status during pregnancy. Obstet. Gynecol.70(4),593-596 (1987).
89. Solomons NW, Ruz M. Zinc and iron interaction: concepts and perspectives in the developing world. Nutr. Res.17,177-185 (1997).
90. Festa MD, Anderson HL, Dowdy RP, Ellersieck MR. Effect of zinc intake on copper excretion and retention in men. Am. J. Clin. Nutr.41(2),285-292 (1985).
91. Porter KG, McMaster D, Elmes ME, Love AH. Anaemia and low serum-copper during zinc therapy. Lancet2(8041),774 (1977).
Hallberg L, Brune M, Rossander L. Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum. Nutr. Appl. Nutr.40(2),97-113 (1986).





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 