Fifty million people in the world have epilepsy, and there are between 16 and 51 cases of new-onset epilepsy per 100,000 people every year.1 A community-based study in southern France estimated that up to 22.5% of patients with epilepsy have drug-resistant epilepsy.2 Patients with drug-resistant epilepsy have increased risks of premature death,3 injuries, psychosocial dysfunction, and a reduced quality of life.4,5 Here, we review recent progress in the understanding and management of drug-resistant epilepsy. Where appropriate, we have also graded the strength of evidence for specific treatments from class I (highest) to IV (lowest), according to guidelines from the American Academy of Neurology; the classification of evidence is described in Table 1 in the Supplementary Appendix (available with the full text of this article at NEJM.org).6
DEFINITION AND CLINICAL RISK FACTORS
The International League against Epilepsy has recently developed a global consensus definition of drug-resistant epilepsy.7 The overall framework comprises two “hierarchical” levels. Level 1 provides a general scheme to categorize the outcome of each therapeutic intervention as either freedom from seizures or treatment failure on the basis of standard criteria. When a patient has had a trial of an antiepileptic drug that is uninformative for determining efficacy, that treatment trial should be regarded to have an undetermined outcome. This occurs, for example, when an antiepileptic drug has been inadequately tried before early discontinuation at a low dosage. This level 1 assessment forms the basis for the level 2 determination, which defines drug-resistant epilepsy as a failure of adequate trials of two (or more) tolerated, appropriately chosen, and appropriately used antiepileptic drug regimens (whether administered as monotherapies or in combination) to achieve freedom from seizures. This definition is based on the observation that if complete seizure control is not achieved with trials of two appropriate antiepileptic drugs, the likelihood of success with subsequent regimens is much reduced.8,9 Although drug resistance may “remit” over time (at a rate of 4% per year among adults and a higher rate among children), seizure relapse is common, suggesting a fluctuating course.10-12 Other consistent clinical predictors of drug resistance include a high number or frequency of seizures in the early phase of the disorder and the presence of a known, often structural cause of the epilepsy, particularly hippocampal sclerosis.9,13
HYPOTHESIZED MECHANISMS
The mechanisms of drug resistance are likely to be variable and multifactorial according to the underlying cause14 and, in theory, to the drug's site of action. Age also seems to affect treatment outcome, with a higher seizure-free rate observed among elderly people than among younger people.15 Major hypotheses of cellular mechanisms under active investigation can be broadly categorized into several groups (Figure 1
FIGURE 1
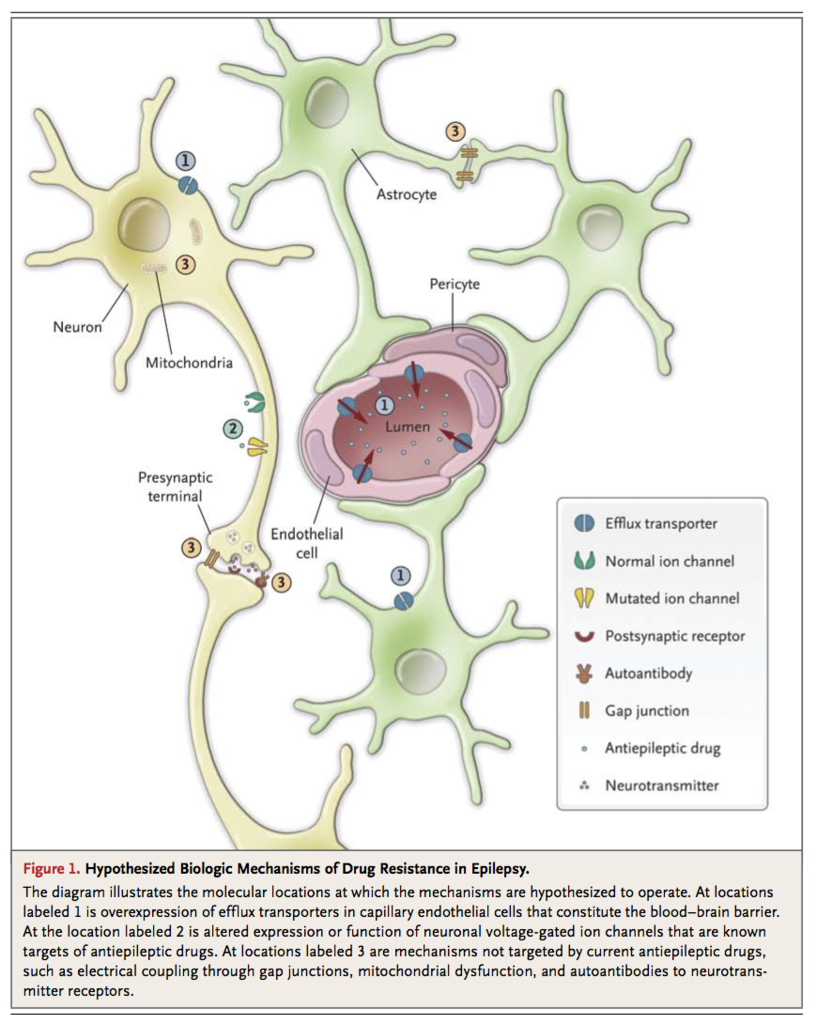
Hypothesized Biologic Mechanisms of Drug Resistance in Epilepsy.
). Most of the studies were performed in adults, and it remains to be determined whether these mechanisms are also relevant to childhood epilepsies.
Failure of Drugs to Reach Their Targets
The “transporter hypothesis” proposes that drug resistance may be attributable to overexpression of multidrug efflux transporters at the epileptic focus. These ATP-binding cassette (ABC) transmembrane proteins extrude substrates from the cell against the concentration gradient. The most extensively studied efflux transporter is P-glycoprotein, which is expressed at a basal, physiologic level in capillary endothelial cells in the brain, where it pumps xenobiotics from intracellular space back to the capillary lumen, thereby maintaining the integrity of the blood–brain barrier and reducing the cerebral accumulation of substrate drugs. In surgically resected brain specimens from patients with drug-resistant epilepsy, up-regulation of P-glycoprotein and other efflux transporters in capillaries, as well as aberrant expression in glial and neuronal cells, has been reproducibly demonstrated.16-18 There is conflicting evidence that polymorphisms of the gene encoding P-glycoprotein (ABCB1) might be associated with a poor response to antiepileptic drug therapy.19,20 Whether human P-glycoprotein transports antiepileptic drugs to a significant extent remains controversial.21 Compelling evidence regarding the clinical relevance of the transporter hypothesis remains lacking.
Alteration of Drug Targets
The “target hypothesis” postulates that alteration in the cellular targets of antiepileptic drugs leads to a reduction in their sensitivity to treatment.22 One study showed that the use-dependent blockade of the fast sodium current in dentate granule cells by carbamazepine was lost in hippocampi resected from patients with carbamazepine-resistant temporal-lobe epilepsy, although this finding did not extend to lamotrigine, which has a pharmacologic action similar to that of carbamazepine.23 Polymorphisms of the SCN2A gene, which encodes the α2 subunit of the neuronal sodium channels, were found to be associated with resistance to antiepileptic drugs in general as well as to those that act on the sodium channels.24 Altered expression of subtypes of the γ-aminobutyric acid type A (GABAA) receptor has also been observed in patients with drug-resistant temporal-lobe epilepsy.25 Whether these changes result in reduced sensitivity to antiepileptic drugs that act on the receptor is unknown. The main weakness of the target hypothesis is its presumption of a working knowledge of the mechanisms of action of antiepileptic drugs, which remain incompletely understood. The hypothesis cannot account for the observation that patients often have epilepsy that is resistant to multiple drugs with different modes of action, although it cannot be ruled out that alteration in drug targets may play a contributory role.
Drugs Missing the Real Targets
Current antiepileptic drugs are intended only to prevent seizures and may not be targeting the appropriate pathogenic processes in some patients. For instance, autoantibodies to ion channels involved in neuronal excitation and inhibition, including voltage-gated potassium and calcium channels,26 and to glutamate N-methyl-D-aspartate (NMDA)27 and γ-aminobutyric acid type B (GABAB) receptors,28 have been identified in patients with seizures of otherwise unknown cause, particularly in the clinical context of encephalitis and sometimes in association with occult cancer. These patients often do not have a response to conventional antiepileptic drugs, and there are conflicting data from uncontrolled studies about whether immunotherapy can be effective.29 Other proposed cellular mechanisms of seizures and epileptogenesis include, but are not limited to, mitochondrial oxidative stress and dysfunction30 and electrical coupling through gap junctions in neurons or even glial cells.31 These mechanisms represent potential novel targets for future drug development.
PRINCIPLES OF MANAGEMENT
Ruling Out Pseudoresistance
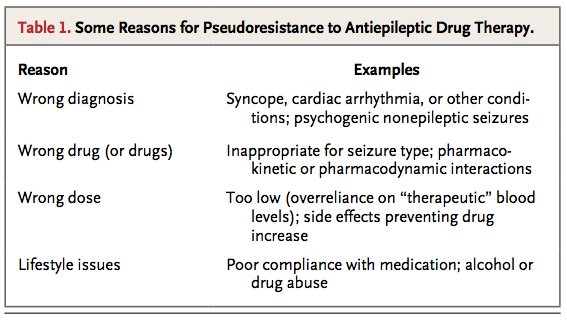
Pseudoresistance, in which seizures persist because the underlying disorder has not been adequately or appropriately treated, must be ruled out or corrected before drug treatment can be considered to have failed. This phenomenon may arise in a number of situations (Table 1
TABLE 1
Some Reasons for Pseudoresistance to Antiepileptic Drug Therapy.
), of which misdiagnosis of epilepsy is probably the most common. Conditions that frequently mimic epileptic seizures include vasovagal syncope, cardiac arrhythmias, metabolic disturbances, and other neurologic disorders with episodic manifestations (e.g., transient ischemic attacks and migraine). Psychogenic, nonepileptic seizures are estimated to account for more than 25% of adult cases of apparently drug-resistant epilepsy.32
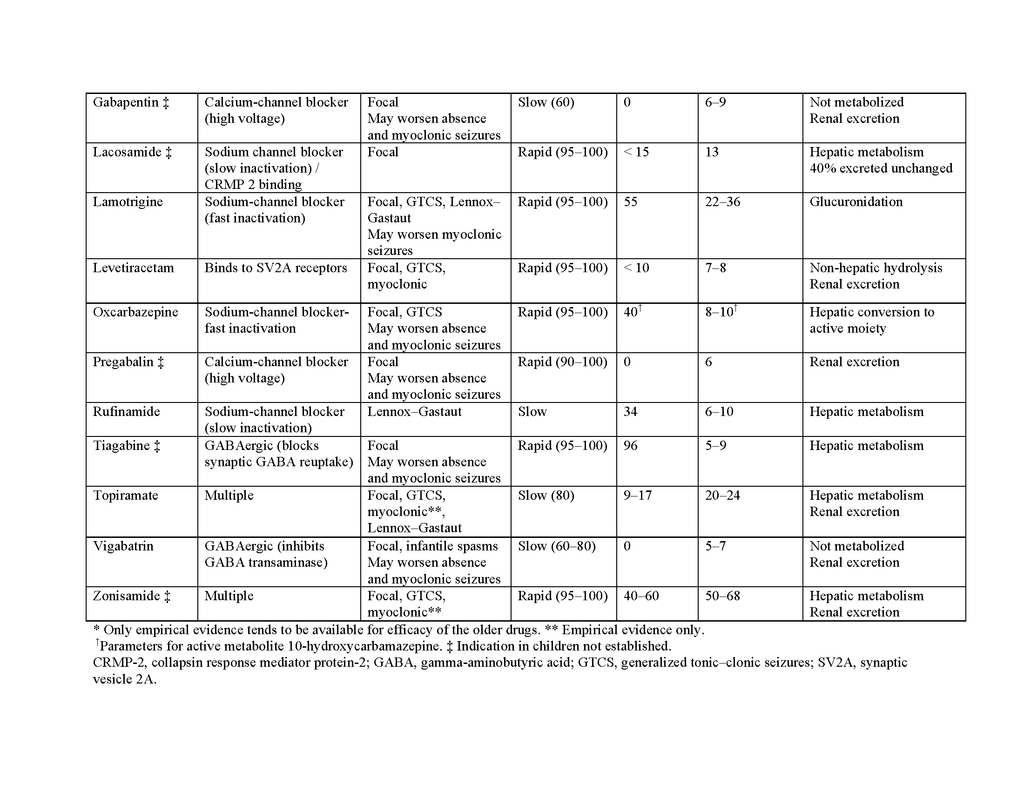
Failure of drug therapy may also result from an inadequate understanding of the pharmacologic properties of antiepileptic drugs, particularly their range of clinical efficacy and pharmacokinetic characteristics. These properties are summarized in Table 2 in the Supplementary Appendix. Because the spectrums of activity vary among antiepileptic drugs, incorrect classification of the syndrome or seizure type can lead to treatment failure or even seizure aggravation. Table 3 in the Supplementary Appendix shows the latest recommendations for seizure classification. Phenytoin, carbamazepine, gabapentin, oxcarbazepine, vigabatrin, tiagabine, and pregabalin can worsen absence epilepsy and myoclonic seizures.33 Lamotrigine can also exacerbate some myoclonic epilepsy syndromes.34 Another problem is that an antiepileptic drug may fail to control seizures satisfactorily because it is not prescribed at the optimal dosage. This may result from an injudicious reliance on monitoring of serum drug concentrations; a “therapeutic range” can be interpreted as dictating dosage adjustment without adequate clinical correlation.35
Other possible causes of pseudoresistance may be related to the patient's lifestyle or behavior, particularly insufficient adherence to the therapeutic regimen, which may even contribute to increased risks of illness and death.36 Abuse of alcohol and recreational drugs can cause seizures. Sleep deprivation and stress are also common seizure-precipitating factors.
General Approach
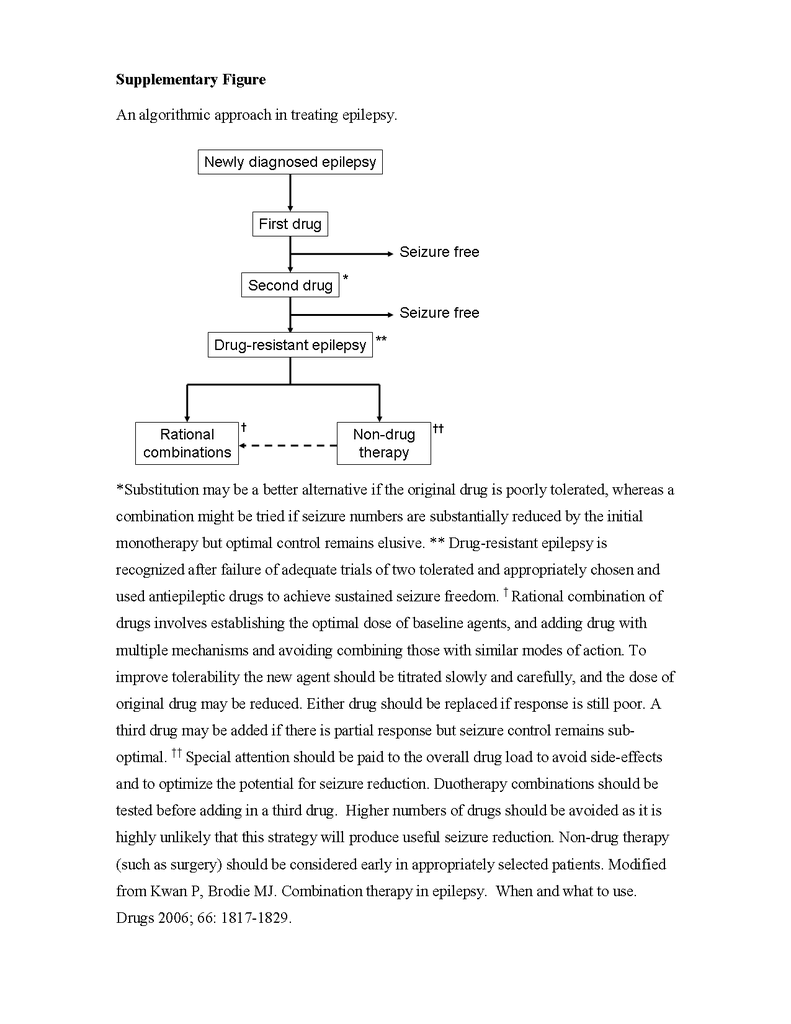
Once a patient's epilepsy is recognized to be drug resistant, a personalized treatment plan should be formulated to limit any cognitive deterioration or psychosocial dysfunction. It is a good idea to mention early in the course of treatment that total freedom from seizures may not be attainable. This approach may help pave the way for a later palliative strategy if that proves to be necessary. Nondrug therapy such as epilepsy surgery should be considered. Patients should also be informed of the risk of sudden, unexpected death in epilepsy; appropriate precautions might include nocturnal supervision, although hard evidence is lacking that this is likely to be lifesaving.37 Conditions commonly associated with treatment-resistant epilepsy, such as anxiety, depression, and cognitive and memory disturbances, should be recognized and treated. An algorithmic approach to treatment is illustrated in the figure in the Supplementary Appendix.
Combination Therapy
Generating robust clinical evidence of suitable combinations of antiepileptic drugs has been challenging because of the large number of possible combinations of drugs and dose ranges. The strongest evidence in favor of synergism comes from nonrandomized, controlled studies involving adults who received a combination of sodium valproate and lamotrigine for partial-onset and generalized seizures (class III evidence)38,39; these observations are supported by animal models.40 Other combinations that are sometimes recommended, largely on the basis of anecdotal reports or studies with small samples, include valproate with ethosuximide for absence seizures (class IV evidence)41 and lamotrigine with topiramate for a range of seizure types (class IV evidence).42
One strategy for combination therapy that has been advocated is a pharmacomechanistic approach based on the drugs' differing modes of action, although high-quality data to support this strategy are lacking. Data from studies in animals suggest that administering two drugs that act on the same pharmacologic pathway, such as sodium-channel blockade, is less effective than administering two drugs with different mechanisms of action.43 The possibility that combining two drugs that act by blocking neuronal voltage-dependent sodium channels may not be clinically useful was first reported in 1975 by Cereghino and colleagues, who treated 47 cognitively impaired patients with phenytoin, carbamazepine, and phenobarbital sequentially and found that combinations with phenobarbital were more effective than phenytoin combined with carbamazepine (class III evidence).44 Deckers and colleagues undertook a comprehensive review of the available data from studies in animals and humans and concluded that combinations involving a sodium-channel blocker and a drug with GABAergic properties appeared to be particularly beneficial.45 However, the successful licensing of the latest sodium-channel blockers, lacosamide and eslicarbazepine, as adjunctive treatment was based on data from studies in which the majority of patients were already taking drugs with similar mechanisms of action, such as carbamazepine or lamotrigine. Combinations of drugs that act primarily by blocking voltage-dependent sodium channels (e.g., lamotrigine and carbamazepine) may be more likely to be associated with neurotoxic effects, such as dizziness, diplopia, and ataxia (class III evidence).38 The suggested strategy for combining drugs is shown in the figure in the Supplementary Appendix.
Latest Developments in Drug Therapy
In double-blind, randomized trials, the efficacy of adjunctive treatment with modern antiepileptic drugs has been disappointingly small,46 fueling continuing efforts to develop new compounds. Although a reduction in seizure frequency of 50% or more is generally accepted as demonstrating efficacy for regulatory purposes, the clinical relevance of such an improvement to the overall health status of patients is limited47 and freedom from seizures should remain the goal of treatment. In the past 2 years, two new sodium-channel blockers, lacosamide48 (in the United States and Europe) and eslicarbazepine49 (in Europe), have been licensed for use in adults with partial seizures with or without secondary generalization (class I evidence). Rufinamide has shown effectiveness as a treatment for the Lennox–Gastaut syndrome in infants and children (class I evidence).50 Vigabatrin was recently licensed in the United States as an adjunctive treatment for complex partial seizures in adults and as monotherapy for infantile spasms in children from 1 month to 2 years of age (class I evidence), although it has been available elsewhere for many years.51 Stiripentol has been approved under the orphan-drug procedure in Europe for the treatment of Dravet's syndrome, a rare childhood epilepsy syndrome (class I evidence).52
The U.S. and European regulatory authorities have recently granted approval to retigabine (ezogabine in the United States and Canada) as an adjunctive treatment for refractory partial seizures with or without secondary generalization in adults (class I evidence).53 Unlike other antiepileptic drugs, this drug acts by opening potassium channels. Other drugs that are undergoing phase 3 trials include brivaracetam (which, like levetiracetam, binds to the synaptic vesicle protein 2A molecule) and perampanel, which modulates glutamate neurotransmission mediated by α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA).54
Nondrug Therapy
Patients who meet the criteria for having drug-resistant epilepsy should be evaluated early for surgical treatment, particularly if they have a surgically remedial syndrome, such as unilateral hippocampal sclerosis or other resectable lesions. 55 The decision to offer surgical treatment requires an individualized risk–benefit assessment that includes consideration of the pros and cons of additional trials of antiepileptic drugs. A range of surgical procedures can be performed, depending on the indication. The prototype is anterior temporal lobectomy, which has been shown in a randomized, controlled trial56 to be superior to continued medication in providing long-term relief from seizures in up to 70% of adults with drug-resistant temporal-lobe epilepsy (class I evidence).57 Other potentially curative procedures include resection of structural lesions (lesionectomy) such as glial tumors and vascular malformations (class III evidence). Even when magnetic resonance imaging reveals no lesions in patients with temporal or extratemporal epilepsy, resection may be supported by findings from functional imaging (ictal single-photon-emission computed tomography or interictal positron-emission tomography) with or without invasive electroencephalographic monitoring, although the outcomes of surgical treatment in such cases tend to be less favorable than those in lesional cases (class III evidence).58
Palliative procedures, which are intended to disrupt the pathways important for the propagation of epileptiform discharges and thus reduce the frequency and severity of seizures, may be considered when resection of the seizure-generating region is not possible. Corpus callosotomy is usually performed in children with clinically significant learning disabilities and severe generalized epilepsy, particularly when the disorder causes atonic seizures that are associated with frequent falls and subsequent injuries; adults may also benefit but to a lesser degree (class III evidence).59 Multiple subpial transection is a less commonly performed procedure that is reserved for situations in which the epileptogenic focus cannot be removed because of close proximity to eloquent cortex.60 This procedure is usually performed in children in conjunction with cortical resection, which makes it difficult to assess its specific efficacy (class IV evidence). Hemispherectomy or functional hemispherotomy, performed in both children and adults, is a more dramatic procedure in which an extensively diseased and epileptogenic cerebral hemisphere is removed or functionally disconnected (class IV evidence).
The vagus-nerve stimulator is a multiprogrammable pulse generator that is implanted in the patient's upper chest and delivers electrical current to the vagus nerve, usually the left nerve, in the neck.61 The device has been approved for use as an adjunctive therapy for adults and adolescents older than 12 years of age whose partial-onset seizures are resistant to antiepileptic medication, although the response is modest (class I evidence).
The ketogenic diet (a high-fat, low-protein, low-carbohydrate diet) is used in children with drug-resistant epilepsy. A randomized, controlled trial showed that the number of seizures fell by more than 50% in approximately half of children after 1 year on the diet (class II evidence).62 The diet seems to be effective for all seizure types. The major problem is adherence to the restrictive (and unpleasant) dietary regimen. Therefore, a modified Atkins diet is under evaluation as a potential alternative in adults and for environments in which strict supervision is unavailable (class IV evidence).63
NEW AND EMERGING THERAPIES
A range of new approaches to the treatment of drug-resistant epilepsy are under active investigation. Those that are in advanced clinical development include technology-based approaches that use intracranial and extracranial treatment systems, which typically provide either electrotherapy or pharmacotherapy and which in some cases may be automatically administered when a seizure is detected by sensors.64 One such intracranial device, which delivers scheduled electrical stimulation bilaterally to the anterior nucleus of the thalamus, was studied in a multicenter, double-blind, randomized trial involving 110 adults with drug-resistant focal epilepsy (class I evidence).65 The group receiving electrical stimulation had a 29% greater reduction in seizures than the control group (no stimulation). After 2 years of open-label use in a continuation phase of the trial, the median reduction in seizure frequency was 56%; 54% of patients had a seizure reduction of at least 50%, and 14 patients were seizure-free for at least 6 months. The Food and Drug Administration Neurological Devices Panel recently recommended approval of the device. Another device undergoing a phase 3 clinical trial in adults involves a “closed-loop system”: when the device detects epileptiform activity, it also delivers electrical stimulation to the site of this activity. The limited published clinical data regarding the device appear to be favorable.66 Other promising therapeutic strategies in more preliminary stages of development include stereotactic radiosurgery, stem-cell therapy, and gene therapy.67,68
COMPLEMENTARY AND ALTERNATIVE THERAPIES
Complementary and alternative medicine encompasses diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine as practiced, for example, in the United States. Such therapies are widely used (up to 50% of people with epilepsy in developed countries may have used them at some time), although patients may be reluctant to spontaneously report their use to physicians.69 The majority of patients use complementary or alternative therapies not for seizure control but for general health purposes or for symptoms that could be indicative of coexisting conditions, such as depression, or of treatment-related adverse events.70 Clinicians should therefore specifically inquire about the use of such therapies and the underlying reasons, which may prompt additional evaluations and treatments.
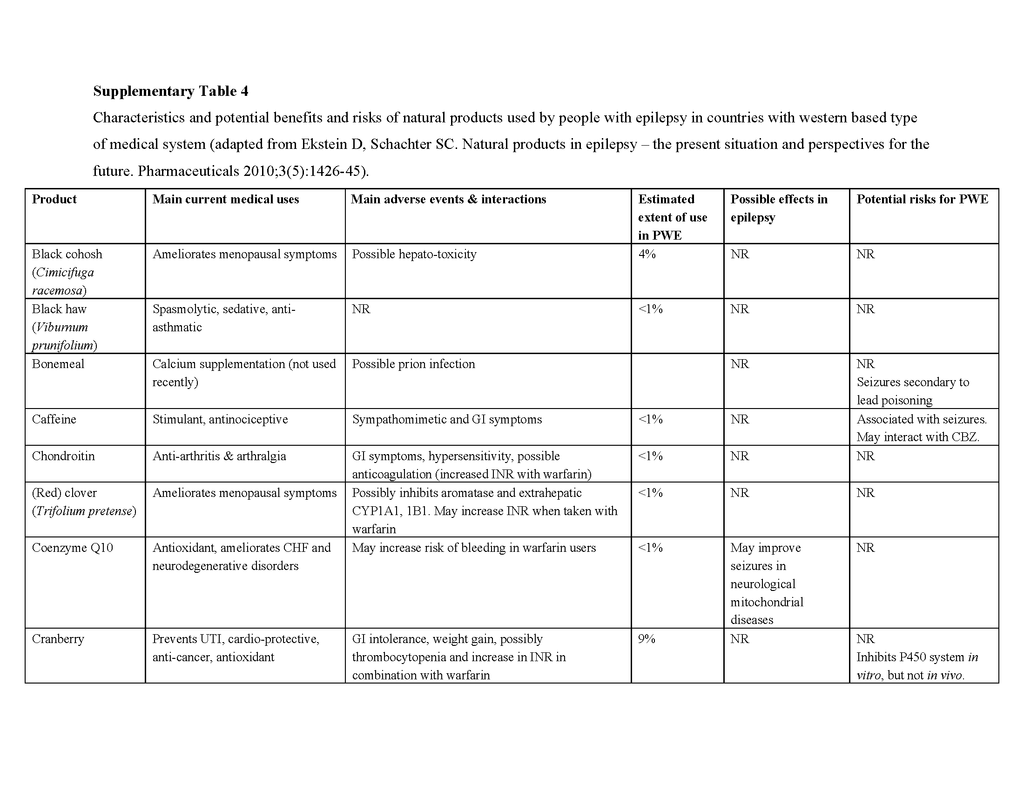
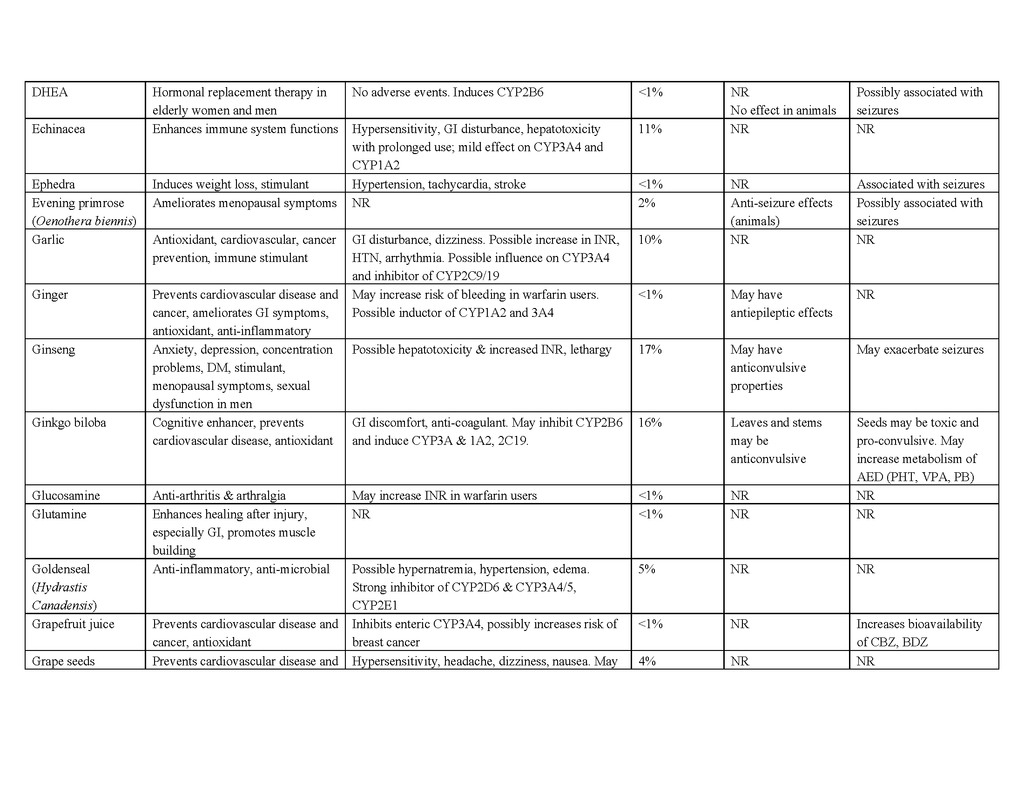
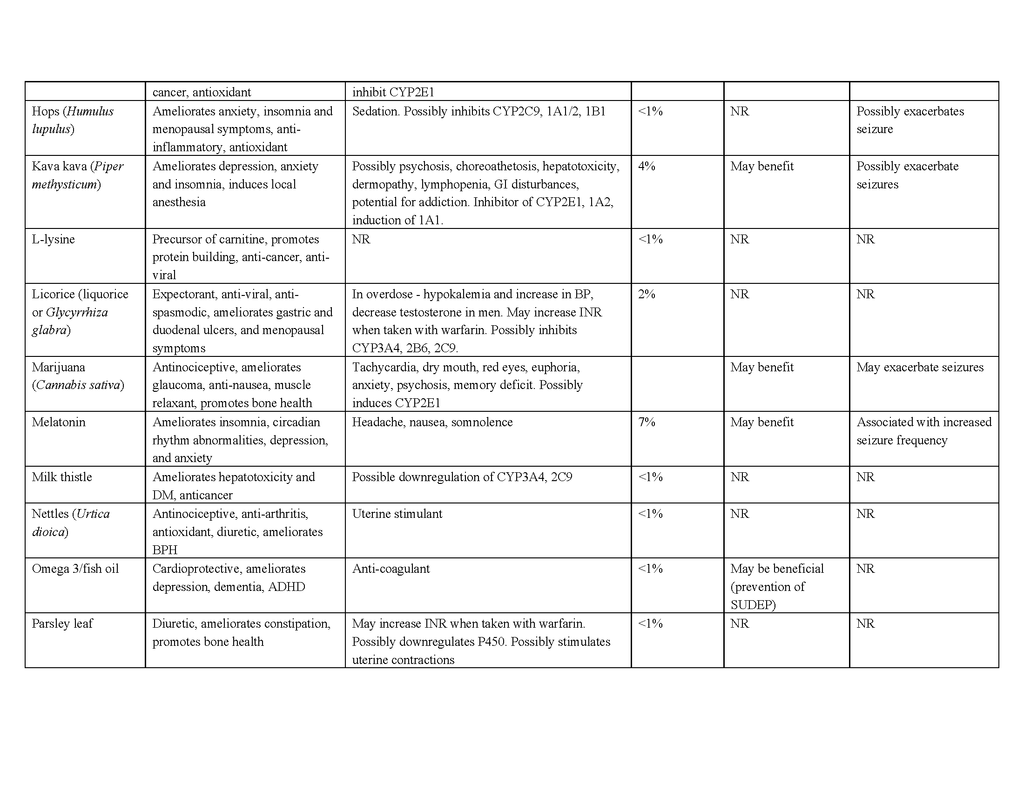
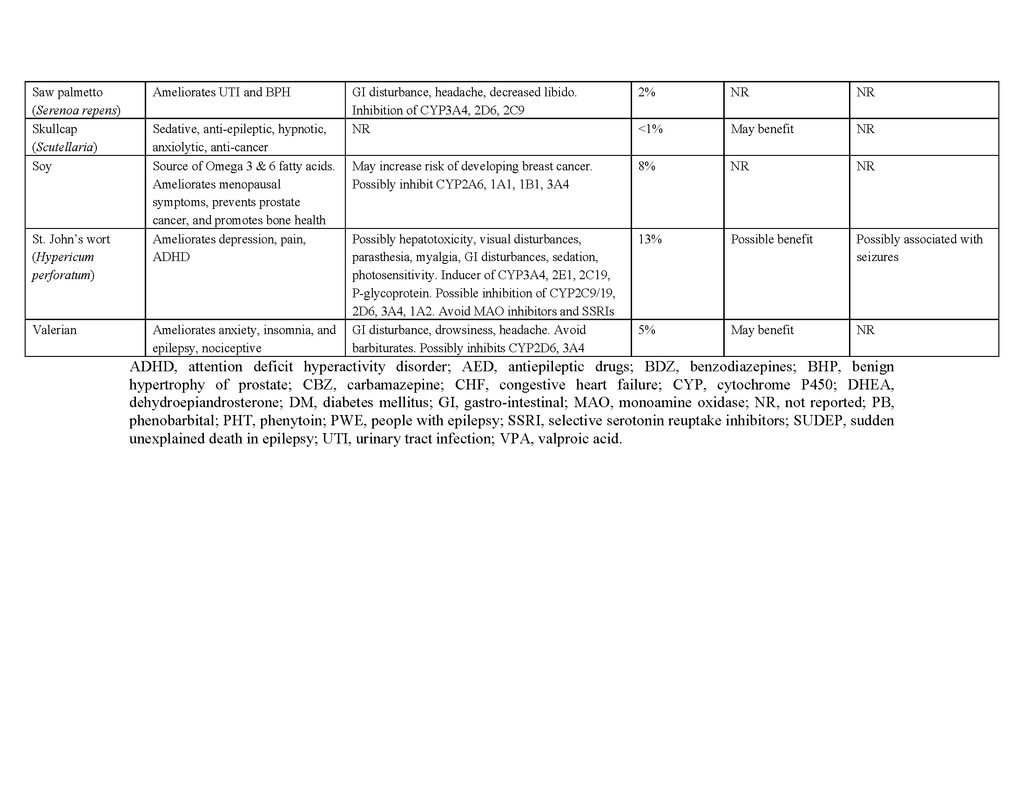
To date, no complementary or alternative therapy has been shown to be effective for epilepsy in multicenter, double-blind, controlled trials. Contrary to the popular belief that “natural is safe,” such therapies can be harmful to people with epilepsy, and natural products pose the greatest risk of side effects, interactions with antiepileptic drugs, and seizure exacerbation.70 Table 4 in the Supplementary Appendix lists the characteristics and potential benefits and risks of natural products that are reportedly used by people with epilepsy. For example, ginkgo biloba, often taken to enhance cognitive function, has been suspected of lowering serum concentrations of phenytoin and valproate by inducing the hepatic drug-metabolizing cytochrome P-450 enzyme CYP2C1971,72 and has anecdotally been reported to exacerbate seizures.73 St. John's wort, commonly taken to relieve symptoms of depression, may also, at least in theory, interact with antiepileptic drugs through induction of cytochrome P-450 enzymes, although data in support of a clinically relevant interaction are lacking.74 Nonetheless, some natural products and their constituent compounds have been shown to have mechanisms of action that are relevant to epilepsy or to have anticonvulsant properties in animal models and are undergoing further preclinical evaluation.75
Dr. Brodie reports receiving consulting fees from Eisai, lecture fees from GlaxoSmithKline, UCB Pharma, Eisai, Novartis, and Medtronic, travel accommodations paid by UCB Pharma, and grant support to his institution from UCB Pharma and Eisai. Dr. Kwan reports receiving consulting fees from Pfizer, lecture fees from Eisai, and travel accommodations paid by UCB Pharma; he also holds a patent related to a rapid HLA typing method (PCT/CN2009/074891), and his institution has received grant support from Eisai, Johnson & Johnson, Pfizer, and UCB Pharma. Dr. Schachter reports receiving consulting fees from Marinus Pharmaceuticals, NeuroTherapeutics Pharma, Sepracor, BrainScope, and Cyberonics, lecture fees from Elsevier and Pri-Med, and payment for serving on a data and safety monitoring board from Quintiles and AXIS Healthcare Communications; he also holds patents related to seizure-detection algorithms and huperzine as a treatment for epilepsy (20100234416, 20060264455, and 20060111644).








 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 