
之前其實也有簡單跟各位提過了:胃輕癱(gastroparesis)
Gastroparesis is a chronic condition of the stomach characterized by a delay in gastric emptying with no mechanical obstruction present. It is estimated to affect up to 4% of the population and is most commonly seen in women and patients with diabetes.1-3Signs and symptoms frequently reported include nausea, vomiting, early satiety, bloating, and upper abdominal discomfort.4Complications such as esophagitis, Mallory-Weiss tears, and bezoars may also result and contribute to morbidity.5,6
Causes
Normally, the vagus nerve in the stomach controls muscle contractility and peristalsis of food into the small intestine. In patients with gastroparesis, this nerve is believed to be damaged, leading to decreased signaling of stomach muscles, yielding a slowed emptying time and perhaps abnormal digestion. This condition may be idiopathic in nature, but is commonly seen in patients with diabetes, neurologic or smooth muscle disorders, anorexia or bulimia, gastroesophageal reflux disease (GERD), infections, endocrine disorders, autoimmune diseases, cancers, and upper gastrointestinal (GI) surgery.6,7 In addition, a number of medications (listed in TABLE 1) have been associated with delayed gastric emptying and reduced intestinal contractility.4,7-9
In most cases, gastroparesis is categorized as being idiopathic, diabetic, or postsurgical. Idiopathic gastroparesis usually affects younger or middle-aged women and can present as an acute viral gastroenteritis, which may be the underlying cause or precipitating factor. In patients with diabetes, gastroparesis is commonly seen in those with other end-organ complications. It is believed that neuropathic mechanisms are responsible for the altered gastric motor function and antral or pyloric contractility (associated with elevated levels of glucose).1,3,7 An estimated 25% to 55% of type 1 and 30% of type 2 diabetic patients demonstrate some degree of gastroparesis.9 In regard to abdominal surgery, various disturbances in anatomy and physiology may result in delayed gastric emptying, as well as in changes in vagal modulation.1,4,9
Diagnosis
Clinical manifestations of gastroparesis warrant a thorough medical history and physical examination. In addition, a number of diagnostic tests may be used to appropriately diagnose this condition including scintigraphy, radiography, breath testing, and antroduodenal manometry.4,7
The gold standard for diagnosis of gastroparesis is gastric emptying scintigraphy of a solid meal. This test is performed up to 4 hours post ingestion of a radiolabeled meal in order to adequately detect delayed gastric emptying by measuring the volume of stomach contents and stomach relaxation.4,10,11
The upper GI barium series is another method for measuring delayed or poor gastric emptying of barium, gastric dilation, and/or the presence of retained food or bezoar. When little or no emptying of the barium occurs at 30 minutes and retention of gastric barium is noted at 6 hours, gastroparesis is most probable. In some cases (mainly research or study related), breath testing using a nonradioactive isotope bound to solid food may be performed to determine and measure gastric emptying.7,11 Antroduodenal manometry may also be utilized to measure motor function, coordination, and relaxation of the stomach and duodenum during fasting and/or postprandial periods. This method can also help to distinguish between neuropathic and myopathic disorders, in addition to aiding in the diagnosis of small bowel obstruction or rumination syndrome.7,8,11
Other methods that are not as frequently used but may be useful in the diagnosis of gastroparesis include electrogastrography, ultrasonography, MRI, single-photon emission computed tomography (SPECT), and satiety testing. An emerging diagnostic tool is the SmartPill, a wireless capsule monitoring system for the GI tract. In this outpatient procedure, a nondigestible capsule travels throughout the GI tract over 3 to 6 hours and records variables such as pH, temperature, and pressure changes. This information is transmitted to a receiver, which determines gastric emptying time, as well as how well food or liquids pass through the intestines. The capsule is ultimately eliminated in the stool within 24 to 72 hours after administration.4,10,11
Management
Following the diagnosis of gastroparesis, clinicians must determine the degree of symptomatology in order to formulate an appropriate treatment plan. Mild gastroparesis may be managed by proper nutrition and weight maintenance, while moderate to severe symptoms may require medication therapy in addition to dietary and lifestyle modifications. In refractory patients, gastric failure may occur and require the aforementioned interventions, as well as percutaneous endoscopic gastrostomy (PEG) tube placement, parenteral nutrition, or gastric electrical stimulation.4,7,8
In all cases, the management of gastroparesis should include three main goals: 1) reduction or prevention of symptoms; 2) correction or prevention of nutritional, fluid, and electrolyte imbalances; and 3) identification and treatment of underlying causes.4
Nutritional Considerations: One of the mainstays of treatment for patients with gastroparesis is dietary modification. Identification and avoidance of foods causing GI intolerance (e.g., carbonated products, dairy, or red meat) is the first step. It is also suggested that solid, high-fiber, and fatty foods may precipitate symptoms, since these food require a longer digestion time. Patients are commonly advised to eat multiple, small meals throughout the day, cook or puree food until softened, and chew thoroughly when eating. In addition, light exercise such as sitting or walking for a period of 1 to 2 hours post-consumption may also be beneficial. In patients with moderate-to-severe gastroparesis, thick or thin liquids may be recommended (e.g., supplemental nutritional drinks) for better tolerance and passage through the stomach.3,4,12,13
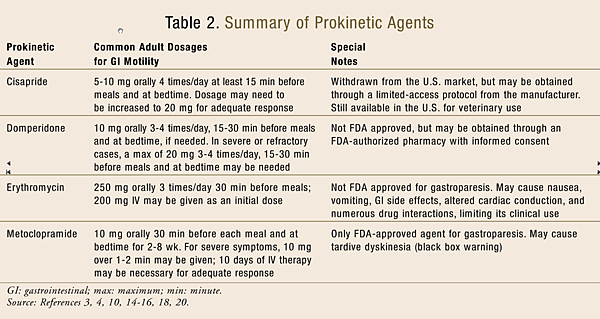
Pharmacologic Therapy: Currently, only a few pharmacologic agents are available for the treatment of gastroparesis (TABLE 2), which can be classified into three main categories: motilin receptor agonists, dopamine receptor antagonists, and serotonin (5-HT4) receptor agonists.
Motilin receptor agonists help to produce antroduodenal contractility for the rhythmic forcing of food and drink through the GI tract. Macrolide antibiotics (erythromycin, azithromycin, and clarithromycin) act through this mechanism, promoting upper GI transit. However, erythromycin has been reported to also cause pyloric relaxation and is the most potent gastric emptying stimulant among all other prokinetic agents. It is important to note that despite its impressive reduction in symptoms, erythromycin can cause nausea, vomiting, abdominal pain, and other GI effects, as well as altered cardiac conduction (e.g., QTc prolongation and ventricular arrhythmias), particularly at higher doses. Caution should also be used with any agents requiring substantial metabolism via CYP3A4 due to potentially significant drug interactions. In addition, tachyphylaxis can result in patients chronically treated with oral erythromycin therapy; therefore, treatment should be initiated at low doses and titrated according to individual patient response.3,4,14-16
Dopamine receptor antagonists act to prevent the dopaminergic inhibition of gastric motility. Metoclopramide and domperidone are the two agents in this class most commonly used in the treatment of gastroparesis. Both drugs have been extensively studied in patients with diabetic gastroparesis. They may also provide antiemetic benefits to patients with gastroparesis as a result of their dopamine receptor antagonism in the brainstem.3,4,17
Metoclopramide is currently the only FDA-approved medication for the treatment of gastroparesis. In addition to its dopamine receptor antagonism, it also acts as a 5-HT4 receptor agonist, stimulating cholinergic neural pathways in the stomach. This medication has been reported to produce significant symptom improvement and gastric emptying acceleration in patients. Common side effects include drowsiness, fatigue, restlessness, irritability, nausea, diarrhea, breast tenderness, galactorrhea, and menstrual irregularities.3,4,10 Troubling cases of tardive dyskinesia have also been reported, prompting the FDA to place the following black box warning on all formulations of metoclopramide: “Tardive dyskinesia: may cause tardive dyskinesia, which is often irreversible; duration of treatment and total cumulative dose are associated with an increased risk. Therapy durations >12 weeks should be avoided (except in rare cases following risk:benefit assessment).”4,18
Domperidone is not currently FDA approved, but it is used in Canada and Europe. Its benefits in gastroparesis are believed to be a result of its antiemetic properties rather than any effects on gastric emptying. Since it does not cross the blood–brain barrier, it is associated with fewer dystonic or other movement side effects and may be useful in patients with various movement disorders. Despite its lack of FDA approval, domperidone may be obtained through an Investigational New Drug Application with local Institutional Review Board Approval, which requires patient informed consent and dispensing from an FDA-authorized pharmacy.3,4,10,19
Cisapride is a 5-HT4 receptor agonist that was initially FDA approved for the treatment of nocturnal heartburn. In addition to alleviating symptoms in GERD patients, this agent also showed some benefits in patients with gastroparesis. It had been noted to stimulate antral contractions, improve antroduodenal coordination, and accelerate gastric emptying as a result of its release of acetylcholine from cholinergic nerves within the GI tract. However, in 2000 the FDA required the manufacturer of cisapride to voluntarily withdraw the product from the U.S. market due to reports of numerous drug interactions, serious cardiac arrhythmias, and associated deaths. The drug is still available for patients who meet certain strict eligibility criteria, but only through a limited-access protocol directly from the manufacturer.3,4,10,20
Emerging therapies for gastroparesis include botulinum toxin and octreotide. Although both have only been studied in a few small clinical trials, these agents have shown some benefits. Botulinum toxin is an agent that blocks overactive nerve impulses by inhibiting acetylcholine, temporarily preventing the contractility of muscles. It is commonly used for the treatment of skin wrinkles, hyperhidrosis, and other neuromuscular disorders, but it has also demonstrated relaxation of pyloric sphincter resistance in patients with gastroparesis, resulting in mild improvements in gastric emptying and modest symptom reduction.4,8,10,21
Octreotide is a long-acting synthetic analogue of endogenous somatostatin that inhibits growth hormone, glucagons, and insulin. In addition, it has been shown to decrease splanchnic blood flow as well as inhibit serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide. Octreotide has been reported to enhance gastric emptying time and reduce bothersome postconsumption satiety. Despite the promise of both botulinum toxin and octreotide, additional studies are needed to further prove their efficacy and safety in patients with gastroparesis.4,10,21
Most recently, a new investigational drug has received fast-track designation from the FDA. TZP-102 is a novel, second-generation ghrelin receptor agonist that is believed to enhance the motility of the GI tract and shows promise in the treatment of gastroparesis as well as other GI disorders. Phase II trials have demonstrated reductions in nausea, early satiety, bloating, and upper abdominal pain in patients with gastroparesis-associated symptoms. However, further clinical evaluations of safety and efficacy are necessary to determine this drug’s benefits and risks for the treatment of gastroparesis and other motility disorders.4,22,23
In certain patients, prokinetic agents may not provide adequate relief of symptoms. Additional pharmacologic options include 5-HT3receptor antagonists, tricyclic antidepressants, muscarinic receptor antagonists, or histamine receptor antagonists for the alleviation of gastroparesis-associated nausea, vomiting, and discomfort. Commonly used adjunctive medications include amitriptyline, dimenhydrinate, desipramine, meclizine, nortriptyline, ondansetron, promethazine, and scopolamine.3,4,10
Nonpharmacologic Therapy: In cases of refractory gastroparesis, gastric electrical stimulation (GES) may be considered to reduce symptoms (especially dyspepsia and vomiting), as well as the need for nutritional supplementation. GES involves the laparoscopic placement of electrodes in the stomach, which are connected to a neurostimulator in a pocket of the abdominal wall. To date, data regarding this intervention and its efficacy/safety profile are limited, requiring further studies to be conducted.3,4,10,24
Surgical procedures may be necessary in certain patients. Individuals with intolerance to other therapies or with associated malnutrition can be evaluated for the placement of venting tubes (e.g., PEG tubes) in order to help reduce symptoms, dehydration, and hospitalizations. In patients with severe, intractable, and refractory gastroparesis who have failed other measures, a gastrectomy may be a final option.3,4,10,25
Role of the Pharmacist
Gastroparesis is a challenging condition to manage appropriately. It affects a substantial number of persons in the U.S. with various underlying conditions and comorbidities. Pharmacists can play a major role in the adequate treatment of gastroparesis by becoming more involved with patient counseling on nutritional recommendations, pharmacologic treatments, and nonpharmacologic interventions. It is important that medication histories and side-effect profiles of each medication also be reviewed thoroughly with patients in order to rule out any similar disorders and avoid any triggers or precipitants of gastroparesis as well as the use of medications that may exacerbate the condition. Encouraging proper lifestyle modifications and explaining the benefits and risks of each medication can help affected patients better understand their condition and how to help alleviate the associated signs and symptoms.
REFERENCES
1. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological profiles, treatment and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
2. Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13:833-844.
3. Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. The American Motility Society Task Force on Gastroparesis. Neurogastroenterol Motil. 2006;18:263-283.
4. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
5. Parkman HP, Schwartz SS. Esophagitis and other gastrointestinal disorders associated with diabetic gastroparesis. Arch Intern Med. 1987;147:1477-1480.
6. Blam ME, Lichtenstein GR. A new endoscopic technique for the removal of gastric phytobezoars. Gastrointest Endosc. 2000;52:404-408.
7. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589-1591.
8. Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820-829.
9. Horowitz M, Harding PE, Maddox AF, et al. Gastric and esophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151-159.
10. Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol Ther. 2008;27:724-740.
11. Parkman HP, Harris AD, Krevsky B, et al. Gastroduodenal motility and dysmotility: update on techniques available for evaluation. Am J Gastroenterol. 1995:90:869-892.
12. Moore JG, Christian PE, Coleman RE. Gastric emptying of varying meal weight and composition in man. Dig Dis Sci. 1981;26:16-22.
13. Emerson AP. Foods high in fiber and phytobezoar formation. J Am Diet Assoc. 1987;87:1675-1677.
14. Weber FH Jr, Richards RD, McCallum RW. Erythromycin: a motilin agonist and gastrointestinal prokinetic agent.Am J Gastroenterol. 1993;88:485-490.
15. Moshiree B, Gupta V, Verne GN, Toskes PP. Azithromycin: a new therapy for gastroparesis. Gastroenterology. 2005;128:A547 (abstract).
16. Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and oral erythromycin. Am J Gastroenterol. 1993;88:203-207.
17. McCallum RW, Ricci DA, Rakatansky H, et al. A multicenter placebo-controlled clinical trial of oral metoclopramide in diabetic gastroparesis. Diabetes Care. 1983;6:463-467.
18. FDA requires boxed warning and risk mitigation strategy for metoclopramide-containing drugs. FDA News Release. February 26, 2009. www.fda.gov/newsevents/ newsroom/pressannouncements/ ucm149533.htm. Accessed November 3, 2011.
19. Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230-1234.
20. Propulsid (cisapride) Dear Healthcare Professional Letter. FDA safety alerts for human medical products. April 2000. www.fda.gov/Safety/MedWatch/ SafetyInformation/ SafetyAlertsforHumanMedicalPro ducts/ucm175000.htm. Accessed November 3, 2011.
21. Fox J, Foxx-Orenstein A. Gastroparesis. American College of Gastroenterology. www.acg.gi.org/patients/gihealth/gastroparesis.asp. Accessed November 3, 2011.
22. TZP-102. Tranzyme, Inc. www.tranzyme.com/products/tzp- 102. Accessed November 3, 2011.
23. Tranzyme Pharma receives FDA fast track status for its oral GI prokinetic drug candidate TZP-102. Tranzyme Pharma. July 27, 2009. http://ir.tranzyme.com/ releasedetail.cfm?ReleaseID= 556387. Accessed November 3, 2011.
24. McCallum RW, Chen JD, Lin Z, et al. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456-461.
25. Jones MP, Maganti K. A systematic review of surgical therapy for gastroparesis. Am J Gastroenterol. 2003;98:2122-2129.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 