N Engl J Med 2012; 366:2492-2501June 28, 2012
This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies is then presented, followed by a review of formal guidelines, when they exist. The article ends with the author's clinical recommendations.
A 50-year-old woman with bipolar depression presents with a widespread pruritic rash of 1 day's duration. She is afebrile and otherwise well. She has a history of childhood eczema and is allergic to sulfonamide antibiotics. Her medications include thyroxine daily, naproxen intermittently, and lamotrigine, which she began taking 3 weeks earlier. How should this case be evaluated and treated?
THE CLINICAL PROBLEM
In the United States, patients fill more than 300 million drug prescriptions and purchase millions of over-the-counter medications each month.1 In many cases patients are using these medications for the first time. Cutaneous reactions are among the most common adverse effects of drugs, including penicillins, cephalosporins, sulfonamide antimicrobial agents, and allopurinol (with an incidence of up to 50 cases per 1000 new users), and particularly the aromatic amine antiseizure medications, including carbamazepine, phenytoin, and lamotrigine (with an incidence of up to 100 cases per 1000 new users).2-7 Drug-related rash is reported for nearly all prescription medications, usually at rates exceeding 10 cases per 1000 new users. These reactions can range from asymptomatic mild eruptions to life-threatening conditions. Cutaneous reactions may be difficult to distinguish from common rashes that are unrelated to medication use, particularly viral exanthems.
Exanthematous drug eruptions (also called morbilliform or maculopapular drug eruptions) are the most common drug-induced eruptions.2,7 They and the much rarer and more serious Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS) are idiosyncratic, T-cell–mediated, delayed (type IV) hypersensitivity reactions.8-11 Classically, antigen-presenting cells present haptens, composed of the drug or its metabolite bound to a protein or peptide, to naive T cells. These antigen-specific T cells proliferate, infiltrate the skin, and release cytokines, chemokines, and other proinflammatory mediators that are responsible for the signs and symptoms of the drug-related rash.12-15 According to an alternative theory known as the p-i (pharmacologic interaction of drugs with immune receptors) concept, small-molecule drugs or their metabolites, which are not complete antigens, activate T cells directly by binding to T-cell receptors.12,13 Irrespective of the mechanism that elicits a T-cell response to a drug, it is not known why only a minority of patients receiving a given drug have a clinical reaction to it, whereas others have immunologic reactivity without a rash.
Alterations in a patient's immune status, as well as genetic factors related to immune response, affect the risk of these drug reactions. Patients with human immunodeficiency virus (HIV) infection, bone marrow transplants, or certain infections for which they are taking particular medications are at especially high risk. 16,17 For example, most patients with infectious mononucleosis who are being treated with aminopenicillins have exanthematous eruptions, as compared with about 5% of patients without this disorder who are taking these drugs. Certain HLA alleles confer a much higher risk of some T-cell–mediated hypersensitivity reactions. Most often described in cases of severe cutaneous reactions, these associations are generally specific to the type of reaction, causative drug, and ethnic group (see Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).18 In Europeans taking carbamazepine, HLA-A*3101 is reported to be associated with an increased risk of maculopapular exanthems.19
Most rashes due to medications are self-limited and only mildly symptomatic. The majority of skin events attributed to drugs are either exanthematous (maculopapular or morbilliform) eruptions (>80%) or urticaria (5 to 10%), but these percentages vary among medications and among patient groups.2,5,20 Among patients who are not immunologically compromised, severe cutaneous reactions to medications are rare (with an incidence of <1 case per 1000 new users), even with high-risk medications.8-11,20-23
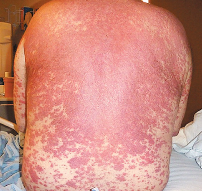
Exanthematous eruptions present as a widespread, symmetrically distributed rash composed of pink-to-red macules and papules that may coalesce to form plaques (Figure 1A, 1B, and 1C
FIGURE 1Clinical Presentations of Common Drug Reactions and Measles.). Although mucous membranes are usually spared, redness without blistering may occur at these sites. Pruritus is frequent but highly variable in severity, and low-grade fever (temperature of <38.5°C) is common.
Urticaria (Figure 1D), photosensitivity, and fixed drug eruptions account for most of the remaining drug-associated eruptions in ambulatory patients. Urticaria shares pathophysiologic features with anaphylaxis and angioedema, both of which may be life-threatening. With most drugs, urticaria is an IgE–mediated, immediate (type I) hypersensitivity reaction. Urticaria due to nonsteroidal antiinflammatory drugs (NSAIDs) or angiotensin-converting–enzyme inhibitors usually reflects the pharmacologic effects of these medications rather than an immunologic reaction.24-26
Photosensitivity eruptions that accompany the use of systemic medications are almost always a consequence of ultraviolet- or visible-light activation of a drug, resulting in phototoxic injury to cells in the skin and a sunburn-like reaction that may blister in exposed areas27 (Figure 1E). Drugs commonly associated with phototoxicity include tetracyclines (particularly doxycycline), thiazide diuretics, quinolones, voriconazole, vemurafenib, amiodarone, and psoralens.28
Fixed drug eruptions present as small (usually <8 cm in diameter), red, round plaques that may sting, usually result in long-lasting pigmentation, particularly in persons with more skin pigment, and typically recur at the same sites (lips, genitalia, and acral skin) on reexposure to the causative medication (Figure 1F).29 Commonly responsible drugs include penicillins, NSAIDs, and acetaminophen.30
STRATEGIES AND EVIDENCE
Evaluation and Diagnosis
In evaluating a patient with a new rash, the clinician should attempt to determine whether the rash is drug-related, whether it is likely to become severe, which medication or medications are most likely to be responsible, which medications can be discontinued, how the eruption should be treated, and what the patient should be told about future medication use. The appearance of the rash (its distribution and morphologic features and whether mucous membranes are involved), the time of its onset relative to the use of drugs, and an assessment of the patient for the presence of fever and other associated symptoms and signs (indicating involvement of other organs) and past reactions to medications, as well as other characteristics of the patient and any coexisting disorders, should guide decision making.
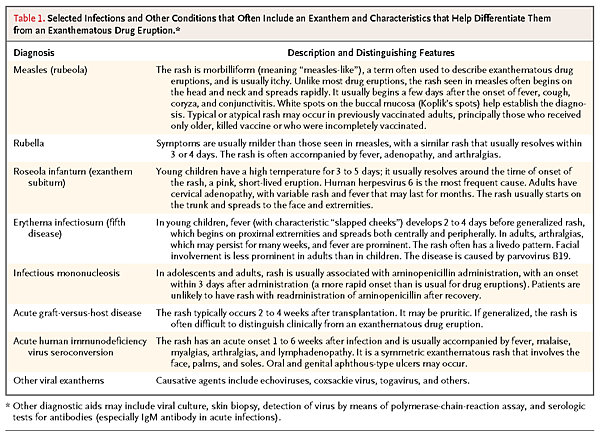
Any new, symmetric exanthematous eruption may be related to medication. Viral exanthems are often difficult to differentiate from drug-induced exanthems (Figure 1G). Viral illnesses are often characterized by the rapid onset of widespread, symmetric eruptions of pink-to-red macules and papules that may coalesce, with fever, malaise, sore throat, and conjunctivitis; however, these features may also be seen with a drug eruption. Viral exanthems are more common in children than in adults and are usually self-limited and mildly symptomatic.31 Table 1
TABLE 1Selected Infections and Other Conditions that Often Include an Exanthem and Characteristics that Help Differentiate Them from an Exanthematous Drug Eruption.describes the characteristic features of some common viral exanthems that help distinguish them from drug eruptions. Patients with fever, sore throat, or malaise due to infections use many medications (particularly antibiotics and NSAIDs) that also cause exanthematous rash. Because of the time required for hypersensitivity to develop in a patient not previously sensitized to a particular drug, a rash that appears within 3 days after the drug has been initiated for these indications is more likely to be due to infection than to the drug.2,14,22
Most drug-induced exanthematous eruptions evolve rapidly, are symmetric and widespread, reach the maximal extent within 2 days after the elimination of the causative drug, and fade within a week after the drug is eliminated. Some drug eruptions start to fade even while the patient is still taking the causative agent. The character of the individual lesions frequently varies according to the body site (e.g., confluent red plaques on the trunk and discrete pink macules and papules on the extremities). The rash is likely to be a deeper red and may even become purpuric in dependent areas. With the exception of patients who bleed easily, one should be able to cause blanching of the rash in nondependent areas. Skin eruptions that differ in appearance from exanthematous drug eruptions are common in patients treated with tyrosine kinase inhibitors (papulopustular eruptions) and patients with hepatitis C who are treated with telaprevir, interferon alfa, and ribavirin (eczematous eruptions).32,33
First-time exanthematous drug eruptions and T-cell–mediated severe cutaneous reactions typically begin to appear 4 to 21 days after the start of treatment with the responsible medication but may develop later in DRESS (Table 2
TABLE 2Features of Selected Severe Cutaneous Adverse Reactions to Drugs.).2,11,22,23 Therefore, assessment of the timing of drug administration relative to the onset of rash and other symptoms is a key step. Resolution after a medication is stopped (known as a “dechallenge”) also helps identify the causative agent.
Since the likelihood of a drug-induced rash varies according to the medication, the population treated, and the indication for use, such factors should be considered in assessing the probability that the patient's rash is due to a specific drug. Aside from the genetic and disease factors discussed above, some groups of patients are at greatly increased risk for unknown reasons. For example, the rate of drug-related rash among young women treated with the antibiotic gemifloxacin (>20%) is about 10 times as high as the rate among other patients treated for the same indications.34 Organ-specific algorithms rather than algorithms that assess drug causality irrespective of the affected organ system may improve interrater reliability in the assessment of the cause of drug eruptions.35 Table S2 in the Supplementary Appendix provides an algorithm, adapted from one validated for SJS–TEN (another T-cell–mediated drug reaction), that may help identify the causative drug in cases of exanthematous drug eruptions,36 although it has not been validated for exanthematous reactions.
Assessing the Likelihood of a Severe Reaction
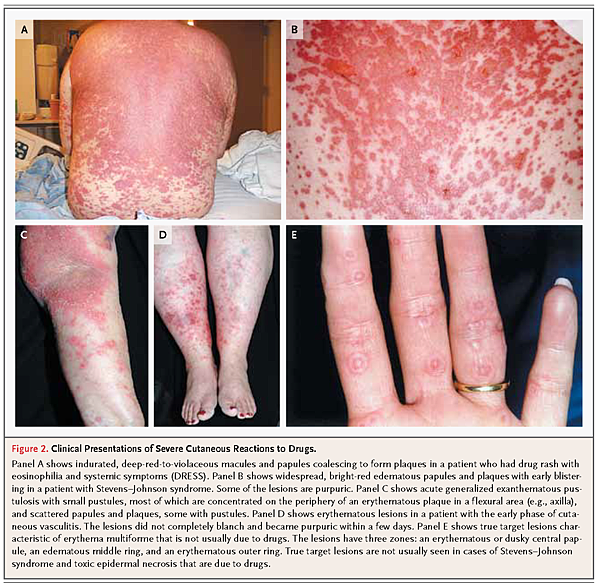
It is important to determine whether an exanthematous drug-induced rash is likely to be an early sign of a severe cutaneous reaction. Determining whether DRESS will develop in a patient with a widespread eruption and fever is particularly challenging. Table 2 summarizes the signs and symptoms associated with medication use for the three severe cutaneous reactions that together account for more than 90% of such reactions: DRESS (Figure 2
FIGURE 2Clinical Presentations of Severe Cutaneous Reactions to Drugs.), SJS–TEN (Figure 2B), and AGEP (Figure 2C). Table S1 in the Supplementary Appendix lists selected medications commonly associated with these reactions, as well as genetic risk factors.
Cutaneous leukocytoclastic vasculitis is characterized by erythematous and purpuric papules predominantly on the lower extremities (Figure 2D). Although most cases are associated with infection or autoimmune disorders, about 20% are due to drugs.37 More than 100 drugs have been implicated, particularly propylthiouracil.38
Serum sickness–like reactions have a variety of cutaneous manifestations, including exanthematous and urticarial eruptions, as well as fever, lymphadenopathy, arthralgia, and inflammation of other organs. Foreign proteins, including biologic agents, minocycline, and cephalosporins, have been associated with these reactions.
Further Evaluation
In most cases of exanthematous drug reactions, a structured clinical evaluation will identify the most likely causative drug (or drugs), which can be withdrawn and avoided in the future (Table S2 in the Supplementary Appendix). Occasionally, greater certainty is needed to establish the causative drug. Whereas in vitro detection of specific IgE antibodies may assist in identifying cases of urticaria, angioedema, and anaphylaxis due to beta-lactam antibiotics and some other drugs, these tests are not relevant to T-cell–mediated drug eruptions, including DRESS and SJS–TEN.39
Various tests have been advocated for establishing the causative drug in cases of exanthematous eruption, but all the tests have limitations. Patch testing has long been used to document the cause of allergic contact dermatitis, a T-cell–mediated delayed hypersensitivity reaction. However, standardized reagents for patch testing are lacking, and sensitivities below 10% have been reported.40 The lymphocyte transformation test attempts to quantify in vitro activation of T cells in response to a drug or its metabolites, but the test is cumbersome and not sufficiently standardized for clinical decision making.40 Drug provocation testing relies on the controlled readministration of a suspected drug to determine causality. Such testing is rarely used in clinical practice because it is not well standardized, may have false positive or false negative results, and carries a risk of triggering a new and possibly more serious drug reaction.
Skin biopsy may help identify SJS–TEN or AGEP in their early phases, but specific histopathological features that would distinguish exanthematous eruptions from DRESS and viral exanthems early in their course are lacking. 41 Phototoxic reactions have characteristic features on biopsy.
Management
Whenever feasible, identification and prompt withdrawal of the suspected drug constitute the cornerstone of management for drug-induced eruptions. This is particularly important for drugs with a short half-life (<24 hours) when an exanthematous rash may represent the early sign of SJS–TEN, since prompt withdrawal of drugs with a short (but not long) half-life has been associated with reduced mortality.42 Patients with signs and symptoms suggesting that the rash may be an early manifestation of a severe reaction should be closely monitored and are often hospitalized until a severe reaction can be ruled out. If the drug is essential and the reaction is not severe, desensitization after recovery may be attempted, but this process is rarely required and is cumbersome.
Sedating antihistamines such as diphenhydramine and hydroxyzine may provide symptomatic relief from pruritus. Potent topical glucocorticoids (which should not be used on the face or in intertriginous areas) may reduce signs and symptoms of the rash, but data from randomized trials of their efficacy in this setting are lacking. Data from a retrospective review and an open-label study, respectively, suggest that early treatment of SJS–TEN with systemic glucocorticoids or cyclosporine is associated with reduced mortality.43,44 The role of intravenous immune globulin in the treatment of SJS–TEN is controversial. The benefits of systemic glucocorticoids relative to their risks in the treatment of exanthematous drug reactions are not clear.
Subsequent Care of Patients with a History of an Exanthematous Drug Reaction
Although in many patients, rechallenge with a drug thought to be responsible for a prior drug-related rash does not result in a new eruption, it should generally be avoided because an eruption on reexposure to the drug may be more severe than the previous eruption. The exception is infectious mononucleosis; if a rash develops in association with the use of aminopenicillin in a patient with this disorder, the risk associated with readministration is only slightly higher than it is for first-time users of the drug.
Exposure to chemically related compounds is also a concern among patients with a prior drug exanthem. However, in many cases, the related drug is tolerated. Among patients who have had an exanthematous (non-IgE–mediated) rash in association with a penicillin antibiotic, the risk of a reaction to a beta-lactam antibiotic is probably less than 10%, and cross-reactivity between cephalosporins with different side chains is infrequent.45 Sulfonamide antimicrobial agents are frequent causes of drug eruptions. The structures of nonantimicrobial sulfonamides, including diuretics, some NSAIDs, and antidiabetic agents, differ sufficiently from the structures of sulfonamide antibiotics that cross-reactivity with sulfonamide antibiotics is unlikely.46 Cross-reactivity is frequent among aromatic amine antiepileptic agents.47 Irrespective of the agent causing an initial drug reaction, persons with a history of drug hypersensitivity are about twice as likely to have hypersensitivity reactions to any other medication as are those without such a history.46
AREAS OF UNCERTAINTY
Limited information suggests that HLA haplotypes and other genetic factors may be useful in predicting the risk of exanthematous reactions to certain drugs, but more data are needed to improve the identification of persons at high risk for such reactions. In addition, a better understanding is needed of factors that mediate differences in the extent and severity of exanthematous drug reactions among affected patients exposed to the same medication. Finally, the usefulness of systemic glucocorticoids and other treatments for exanthematous drug reactions remains uncertain.
GUIDELINES
Guidelines for the identification and management of cutaneous drug reactions have been published by the American Academy of Dermatology (most recently in 1996)48; the American Academy of Allergy, Asthma, and Immunology49; and the British Society for Allergy and Clinical Immunology.50The British guidelines put greater emphasis on skin testing to determine causative drugs than do the recommendations presented here, which are otherwise consistent with these guidelines.
CONCLUSIONS AND RECOMMENDATIONS
The patient described in the vignette almost certainly has an exanthematous drug eruption due to lamotrigine. Fortunately, she has no signs or symptoms suggestive of a severe cutaneous reaction, but she should be informed that if fever, mucosal symptoms, blisters, or malaise develops, she should seek immediate medical attention. She should also be advised to stop taking lamotrigine and to ask her psychiatrist to prescribe an alternative agent that is not an aromatic amine. Since lamotrigine has a long half-life, the patient should be informed that the eruption may take a week or longer to fade. I would recommend that she apply emollients and take sedating antihistamines at bedtime. If the rash is very itchy, I would recommend treatment with a potent topical glucocorticoid for 1 week; although data from randomized trials are lacking, clinical experience suggests that this treatment should reduce secondary skin inflammation and pruritus. Oral glucocorticoids are not indicated, and no further tests are necessary. She should be counseled to avoid this drug and other aromatic amines, including phenytoin and carbamazepine.
Dr. Stern reports receiving consulting fees for the assessment of skin reactions associated with drugs under development from Vertex, InterMune, Johnson & Johnson, Boehringer Ingelheim, and Takeda and its Millennium Division, and for serving on a drug-safety panel for Takeda and as an expert witness in product liability litigation relating to skin reactions for Johnson & Johnson and Mutual, as a consultant to Audet Partners in litigation unrelated to cutaneous drug reactions, and as an expert in patent litigation for Nycomed. No other potential conflict of interest relevant to this article was reported.








 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 