This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies is then presented, followed by a review of formal guidelines, when they exist. The article ends with the author's clinical recommendations.
THE CLINICAL PROBLEM
Asthma is a chronic inflammatory disease of the airways that is characterized by variable narrowing of the airways and symptoms of intermittent dyspnea, wheezing, and nighttime or early-morning coughing. Asthma is a major health problem throughout the world, affecting an estimated 315 million persons of all ages.1 The prevalence of asthma varies widely among countries, ranging from 2% in Vietnam to 27% in Australia.1 Asthma occurs more frequently in adults than in children and more frequently in boys than in girls; however, after the teenage years, asthma occurs more frequently in women than in men. 2
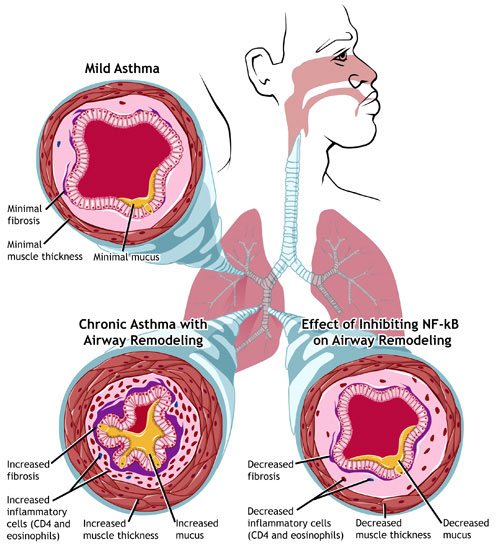
Asthma is clinically heterogeneous, and its pathophysiology is complex.3 Airway eosinophilic inflammation is typical, but many patients with mild asthma have persistently noneosinophilic disease.4 Airway hyperresponsiveness is a consistent feature; irreversible airflow obstruction develops in some patients, presumably as a consequence of remodeling of the airway wall.3 Short periods of loss of asthma control may occur as a result of exposure to nonspecific “triggers,” such as fumes, strong smells, or exercise. Moderate or severe exacerbations are usually due to exposure to allergens or viruses, particularly human rhinovirus.5
The development of asthma in children is influenced by genetic predisposition as well as by environmental factors, including viral infection and sensitization to aeroallergens (e.g., house dust mites or animal dander).6 Altered repair responses of the airway epithelium to these insults lead to inflamed airways, altered smooth-muscle function, and increased production of mucus.3 Persons who are born and raised on a farm have a reduced risk of allergy and asthma, probably because they have been exposed to a wide variety of microorganisms. 7 Risk factors for the development of asthma in middle-aged and older adults are diverse and include work-related exposures (e.g., isocyanates or cleaning products) and lifestyle factors (e.g., smoking or obesity).8,9
KEY CLINICAL POINTS
CONTROL OF ASTHMA
-
Most patients with asthma have mild, persistent disease, which tends to be underdiagnosed, undertreated, and inadequately controlled.
-
The diagnosis of asthma is based on the presence of symptoms of dyspnea, cough, and wheezing and objective confirmation of variable airflow limitation that is at least partially reversible.
-
For mild, persistent asthma, regular controller treatment with low-dose inhaled glucocorticoids and rescue treatment with short-acting beta2-agonists, as needed, is recommended as the initial treatment.
-
If asthma control is not achieved within 3 to 4 months, maintenance treatment should be stepped up with the addition of a second controller medication (long-acting beta2-agonist or leukotriene modifier) or with an increase in the dose of inhaled glucocorticoids.
-
Ongoing patient education, written action plans, and regular follow-up visits to reassess asthma control and adjust therapy are integral to successful management.
Natural History
The natural history of asthma varies considerably according to the age of the patient at the first onset of symptoms, the severity of the asthma, and the patient's sex.8 Mild asthma in children rarely progresses to severe disease, but severe and frequent wheezing is a well-established risk factor for persistence and severity of the disease in adulthood. Boys with asthma are more likely to “grow out” of their asthma, whereas asthma in girls is more likely to persist. Irreversible airflow limitation develops in some patients, particularly in those who have severe exacerbations.10
Severity and Control of Asthma
The clinical spectrum of asthma ranges from mild, intermittent symptoms to severe, refractory disease with frequent exacerbations, but the majority of patients with asthma have mild disease.11Without treatment, these patients have symptoms less than once a day, nighttime sleep disturbances less than once a week, sporadic exacerbations requiring treatment with oral glucocorticoids, and normal lung function between asthma episodes.12,13 Because many patients with mild asthma do not seek care for their symptoms, mild asthma tends to be underdiagnosed, undertreated, and inadequately controlled.14
International expert panels have agreed that achievement and maintenance of good control should be the cornerstone of asthma management.12,13 Asthma control involves the control of symptoms and functional impairments, as well as the reduction of the risk of future adverse events (e.g., asthma exacerbations, adverse effects of medications, and decline in lung function).15 A classification of asthma severity that is based on the intensity of the treatment required to achieve good asthma control has been proposed,16 although it has not been universally accepted.13According to this classification, “mild” asthma refers to asthma that can be well controlled with low-intensity treatment, regardless of the severity of symptoms and airflow obstruction at presentation.
STRATEGIES AND EVIDENCE
Evaluation
The diagnosis of asthma is based on the presence of typical symptoms and on objective confirmation of variable airflow limitation that is at least partially reversible.12,13 The assessment includes a detailed history and physical examination. Wheezing on auscultation suggests asthma, but that finding may be absent during quiet respiration or may be audible only during forced expiration or after exercise. Spirometry or measurement of peak expiratory flow provides an assessment of the severity of airflow limitation, its variability, and its reversibility (Table 1
).12,13
For patients with normal lung function, a negative bronchoprovocation test with inhaled methacholine or a negative test during exercise may be useful for ruling out asthma. Skin testing or in vitro testing is recommended to identify indoor allergens that may contribute to asthma symptoms in patients with relevant exposures.
Once a diagnosis of asthma is made, the level of asthma control should be assessed. Standardized assessments such as the Asthma Control Questionnaire and the Asthma Control Test (see Fig. S1 in theSupplementary Appendix, available with the full text of this article at NEJM.org) have been shown to be valid and reliable tools in clinical practice for distinguishing between asthma that is well controlled and asthma that is not well controlled.17
Patient Education
Patients and health care professionals should discuss and agree on the goals of treatment and develop an individualized action plan. A review of randomized trials of written action plans showed that plans that based the action point on the patient's personal best peak expiratory flow (e.g., action taken when a 15% to 30% decrement is noted), and that included instructions for recognizing early signs of worsening asthma and taking action in case of an exacerbation (including recommendations for the use of inhaled and oral glucocorticoid therapy), resulted in reduced hospitalizations for asthma.18 Ongoing patient education and regularly scheduled follow-up visits to reassess asthma control, adjust therapy, and promote adherence to medication are crucial for long-term success.
Avoidance of Triggers
Relevant triggers that have been shown to aggravate asthma should be addressed and removed if possible. Exposure to aeroallergens has been convincingly shown to worsen asthma control in sensitized patients,19 but there is conflicting evidence about whether measures to reduce exposure to dust mites and pet dander, the most common indoor allergen sources, lead to better asthma outcomes. Randomized trials of single chemical or physical methods that are aimed at reducing dust-mite allergens, for example, have shown no significant effect on asthma symptoms.20 In addition, the clinical effectiveness of removing pets from the home remains unproven. However, multicomponent home-based interventions that target multiple triggers have been shown to reduce asthma symptoms and asthma-related utilization of health care resources.21
Exposure to tobacco smoke increases the severity of asthma in children and adolescents,22 and active smoking reduces the response to asthma medication23 and causes long-term impairment of lung function.24 Smoke-free policies have been associated with a reduction in emergency hospitalizations for asthma. 25 Smoking cessation is therefore strongly recommended for all patients with asthma and their caregivers who smoke.
Coexisting conditions such as rhinitis and obesity may aggravate asthma symptoms, and treatment of these conditions in patients with asthma reduces asthma symptoms and improves lung function.26 Gastroesophageal reflux is also common in patients with uncontrolled asthma; however, in a large, randomized, controlled trial, treatment with proton-pump inhibitors did not result in better asthma control. 27
Asthma Medications
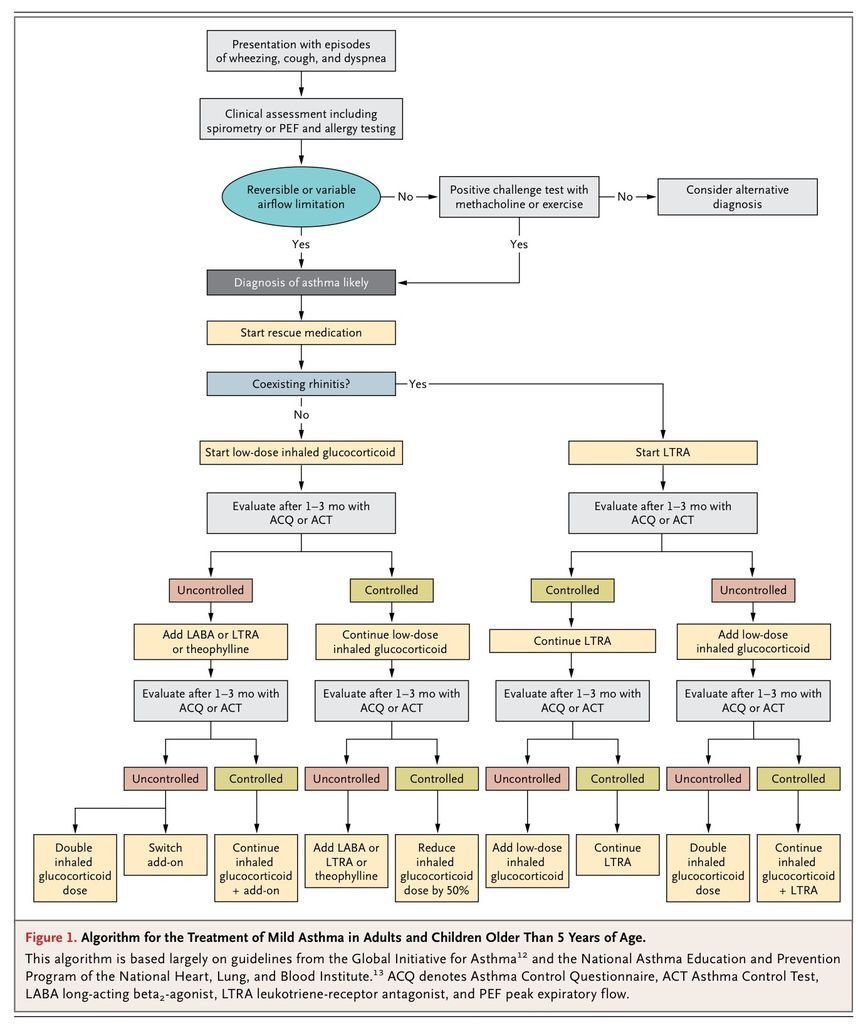
The goal of asthma treatment is to achieve long-term clinical control while minimizing the side effects of treatment. Current guidelines recommend a stepwise approach to pharmacologic treatment,12,13 in which treatment is initiated and adjusted (higher or lower) on the basis of ongoing assessments of the patient's level of asthma control (Figure 1
).
Asthma medications are classified into two categories: medications for long-term control, which are taken on a regular basis to achieve and maintain control of persistent asthma, and quick-relief medications that are used infrequently, when needed, to treat acute symptoms and exacerbations. Most asthma medications are administered through an inhaler, either a metered-dose inhaler with a spacer or a dry-powder inhaler.
For patients with mild, persistent asthma who have not received previous treatment, current guidelines recommend as the initial treatment a regular controller medication with a rescue medication as needed (Table 2
).
Rapid-acting inhaled beta2-agonists are the rescue medications of choice and should be taken as needed to reverse bronchoconstriction and relieve symptoms.28 Although short-acting inhaled anticholinergic agents are not approved by the Food and Drug Administration (FDA) for this indication, they may be used as alternative, albeit less effective, bronchodilators in patients who have unacceptable side effects with beta2-agonists.12,13(Ipratropium bromide, a commonly used anticholinergic agent, has never been considered for centralized approval by the European Medicines Agency; however, it is approved for the treatment of asthma in many European countries.)
For the long-term control of asthma, inhaled glucocorticoids are the most potent and consistently effective medications for achieving overall treatment goals.29 These drugs are therefore the preferred therapy for patients with persistent asthma, including those who have generally well-controlled asthma but have had two or more asthma exacerbations requiring oral glucocorticoid therapy in the previous year.12,13 Constant low-dose inhaled glucocorticoid therapy12,13 has been shown in randomized, placebo-controlled trials to reduce asthma symptoms, improve asthma control and quality of life, improve lung function, diminish airway hyperresponsiveness, control airway inflammation, and reduce the frequency and severity of exacerbations.29,30 A large, nested, case–control study showed that persons who regularly used low-dose inhaled glucocorticoids had a reduced risk of death from asthma.31 Current evidence does not support the assumption that in children, early intervention with an inhaled glucocorticoid may alter the underlying severity or progression of the disease.32 Inhaled glucocorticoids do not cure asthma, and when they are discontinued, asthma control deteriorates in weeks to months in roughly half the patients. 33
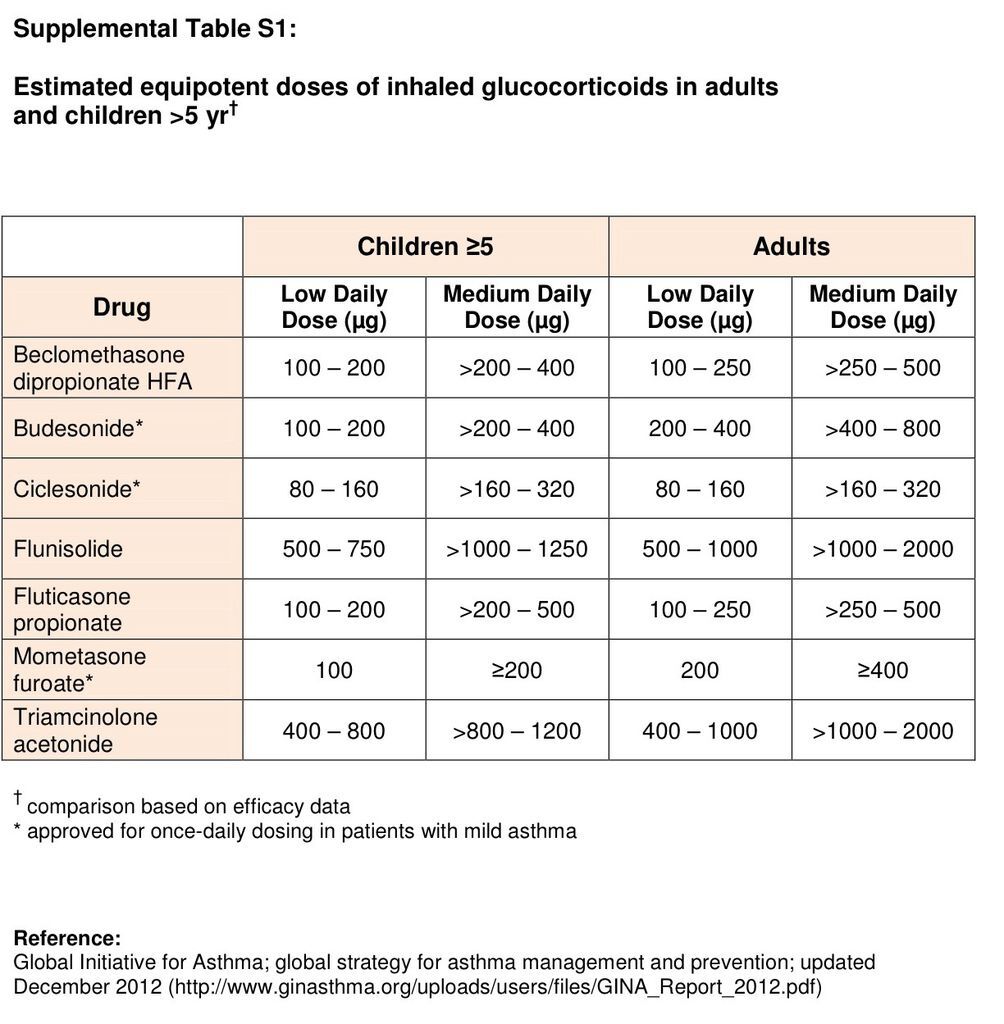
Inhaled glucocorticoids at low doses, even when taken for extended periods of time, are generally considered to be safe.34 Local side effects include dysphonia and oral candidiasis, which may be reduced by rinsing the mouth and spitting after inhalation, using a spacer device, or using extra-fine particle formulations that reduce oropharyngeal deposition.35 Inhaled glucocorticoids are absorbed from the lung into the bloodstream and may cause systemic side effects in susceptible patients; a follow-up assessment of children who were enrolled in a placebo-controlled, randomized trial of inhaled glucocorticoid treatment showed a decrement of 1.2 cm, on average, in final height with long-term treatment. 36 These medications should be adjusted to the lowest dose that is effective in maintaining good asthma control (see Table S1 in the Supplementary Appendix).
Over the past few years, the importance of regular glucocorticoid treatment for patients with mild asthma, as suggested by asthma treatment guidelines, has been challenged. A randomized, controlled trial involving adults suggested that intermittent short courses of inhaled glucocorticoids at the onset of asthma exacerbations have effects similar to those obtained with the regular use of inhaled glucocorticoids with respect to various measures of asthma control. However, not all drugs were similar, and some provided more improvement with daily therapy.37 A recent meta-analysis of four trials involving pediatric patients and two trials involving adult patients showed that daily use of inhaled glucocorticoids is modestly superior to intermittent use with respect to good asthma control, reduction in airway inflammation, improvement in lung function, and reduction in the need for rescue medication.34 Physicians and patients should consider the risks and benefits of each treatment option.
Leukotriene modifiers are alternative medications for the long-term control of asthma.38 These drugs are particularly appropriate for patients who have concomitant allergic rhinitis39 or who have unacceptable local side effects with inhaled glucocorticoid therapy or an inadequate response to inhaled glucocorticoids.40 Large, randomized trials comparing leukotriene-modifier therapy with low-dose inhaled glucocorticoids in adults and children with mild, persistent asthma have shown that as compared with leukotriene-modifier therapy, inhaled glucocorticoid therapy results in significantly greater improvements with respect to most measures of asthma control.41 However, two large pragmatic trials performed in community settings showed that leukotriene-receptor antagonists were similar in efficacy to inhaled glucocorticoids as first-line controller therapy.42
Other options such as theophylline and cromones (nedocromil sodium and sodium cromoglycate) are available, but these drugs are less effective than inhaled glucocorticoids or leukotriene modifiers as initial controller medications for mild, persistent asthma and are therefore not preferred therapies.12,13
Specific Immunotherapy
Although specific immunotherapy has been shown to be effective in reducing asthma symptoms and airway hyperresponsiveness in patients with a single, well-defined, clinically relevant allergy,43the effect of this approach as compared with other treatment options is relatively modest. Specific immunotherapy should therefore be considered only when there is a substantial allergic contribution to a patient's symptoms, and pharmacologic intervention and avoidance of environmental allergens have failed to control the asthma.12,13
Monitoring and Adjustment of Therapy
Once asthma control has been achieved, ongoing monitoring is essential to maintain control and to establish the lowest effective dose of treatment. Inhaler technique should be reviewed regularly (see the video in Hendeles et al.,44 also available with this article, at NEJM.org), and patients should be queried regarding side effects of the medication. Avoidance of triggers and adherence to the medication should be discussed routinely. If the monitoring of peak expiratory flow is included in the written asthma action plan, the patient's personal best peak expiratory flow should be used as the reference value.18
In case of an anticipated brief loss of control (e.g., due to short-term exposure to an allergen), quadrupling the dose of inhaled glucocorticoids for 5 to 10 days might be an effective strategy for preventing progression to a severe exacerbation.37 If lack of control persists for prolonged periods of time (2 to 3 weeks), maintenance treatment should be stepped up with the addition of a second controller medication (a long-acting beta2-agonist or a leukotriene modifier) or with an increase in the dose of inhaled glucocorticoids.45 Randomized, controlled trials have shown that in adults, the addition of a long-acting bronchodilator to inhaled glucocorticoids leads to greater improvement in lung function and quality of life than the addition of a leukotriene-receptor antagonist.46 However, large, randomized, double-blind clinical trials have suggested that long-acting bronchodilators may be associated with increased risks of serious asthma exacerbations and asthma-related deaths.47Therefore, the FDA now advises against using long-acting beta-agonists in patients whose asthma is adequately controlled with low-dose or medium-dose inhaled glucocorticoids.48 In addition, some patients have a better response to the addition of a leukotriene-receptor antagonist.46
AREAS OF UNCERTAINTY
Single-inhaler combination therapy, consisting of an inhaled glucocorticoid and a long-acting beta2-agonist, is increasingly being used for the treatment of patients with moderate asthma.49 Whether this strategy is effective and safe in patients with mild asthma remains to be investigated.
When adequate control of asthma is maintained at a low dose of inhaled glucocorticoids for 3 to 4 months, guidelines recommend stepping down therapy. Proposed step-down strategies include a switch to intermittent use of inhaled glucocorticoids,33 regular use of a leukotriene-receptor antagonist, or once-daily single-inhaler combination therapy with a glucocorticoid and a long-acting beta2-agonist.50 However, the most appropriate step-down strategy remains uncertain.
Better strategies are needed for diagnosing and classifying asthma and for guiding asthma treatment. The use of biomarkers such as sputum eosinophil counts and exhaled nitric oxide levels has been suggested to aid in the assessment of asthma control, but the role of these biomarkers in clinical practice remains uncertain.51
Severe asthma exacerbations can occur even in patients with mild asthma that is well controlled,5with the exacerbations most often provoked by viral infections. Since inhaled glucocorticoids do not fully regulate virus-associated exacerbations, other (preventive) strategies are needed.5
GUIDELINES
The Global Initiative for Asthma (GINA)12 and the National Asthma Education and Prevention Program (NAEPP) of the National Heart, Lung, and Blood Institute,13 among other groups, have published guidelines on the evaluation and management of asthma. The recommendations in this review are generally consistent with the GINA and NAEPP guidelines.
CONCLUSIONS AND RECOMMENDATIONS
The patient described in the vignette has symptoms indicative of mild, persistent asthma. The frequency of daytime and nighttime symptoms and impairment with exercise suggest inadequate control. Before initiating daily controller treatment, I would confirm the diagnosis of asthma by means of spirometry or, in the absence of reversibility, by means of a challenge test with inhaled methacholine. I would also evaluate the patient's sensitization and exposure to allergens and educate him regarding strategies for good control of asthma, including a written action plan. In accordance with current guidelines, I would initiate treatment with regular low-dose inhaled glucocorticoids and a short-acting beta2-agonist as needed for rescue use. If after 3 to 4 months the response to treatment is unsatisfactory (as assessed with the use of a questionnaire such as the Asthma Control Test), I would increase the dose of inhaled glucocorticoids or add a second controller medication. Long-acting beta2-agonists are the preferred therapy to add, but given his atopy and exercise-induced symptoms, a leukotriene-receptor antagonist would be a good alternative. If, however, he has a good response to low-dose inhaled glucocorticoids at follow-up, I would reduce the dose of inhaled glucocorticoids to the lowest possible dose required to maintain asthma control.








 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 