US Pharm. 2013;38(9):HS12-HS16.
ABSTRACT: Many different types of infections can occur during pregnancy. Common infections include sexually transmitted diseases (syphilis, gonorrhea, chlamydia, herpes), urinary tract infections, group B streptococcal disease, listeriosis, and toxoplasmosis. A number of antimicrobial therapy regimens may be used during pregnancy. Pharmacists are in a key position to improve outcomes for pregnant women with any of these infections through recommendations for antimicrobial therapy, monitoring for adverse effects, prevention of drug interactions, and counseling on adherence. Counseling points for pregnant women include appropriate preventive measures, such as lifestyle modifications.
An infection that occurs during pregnancy not only may cause harm to the mother, but may also affect her fetus (or newborn) or her partner.1 This article focuses on selected infections that may occur during pregnancy, including sexually transmitted diseases (STDs), urinary tract infections (UTIs), group B streptococcal disease (GBSD), listeriosis, and toxoplasmosis.
STDs
Syphilis: A bacterial infection caused by Treponema pallidum, syphilis affects approximately 2 million pregnant women worldwide.2 If untreated during pregnancy, syphilis can cause spontaneous abortion, late-term stillbirth, low birthweight, neonatal death, or congenital disease.2
Since early identification of infection is essential to prevent fetal harm and complications, it is recommended that all pregnant women be routinely screened with a serologic test for syphilis at the first prenatal visit, with repeat testing during the third trimester and at delivery for those who remain at increased risk for infection.1
Parenteral penicillin G (PCG) is the only agent with documented efficacy against syphilis during pregnancy. Therefore, all pregnant women with syphilis should be treated with PCG according to stage of infection (TABLE 1). Desensitization is recommended for patients who are allergic to penicillin.1 The Jarisch-Herxheimer reaction (fever, chills, myalgia, headache, hypotension, and tachycardia) occurs in approximately 45% of pregnant women treated for syphilis.3 Although this reaction typically starts within hours of treatment and resolves 24 to 36 hours later, it can cause severe uterine contractions, premature labor, or fetal distress during the second half of pregnancy.2,3 Therefore, pregnant women should be counseled to seek immediate medical attention after syphilis treatment if fever, contractions, or decreased fetal movement develops.1
Gonorrhea and Chlamydia: Gonorrhea, a bacterial infection caused by Neisseria gonorrhoeae, affects about 13,000 pregnant women annually.4 Chlamydia, caused by the bacterium Chlamydia trachomatis, is responsible for infections in approximately 100,000 pregnant women each year.4 If untreated during pregnancy, these infections can cause premature labor, uterine infection, or congenital infection in the form of conjunctivitis, pneumonia, or disseminated disease.4,5
To prevent complications and neonatal infection, it is recommended to routinely screen all pregnant women for C trachomatis and to screen those at risk for N gonorrhoeae during the first prenatal visit, with repeat testing during the third trimester—for chlamydia in patients aged 25 years or less, and for both chlamydia and gonorrhea in those remaining at increased risk for infection.1
Since patients infected with N gonorrhoeae are frequently coinfected with C trachomatis, those treated for gonococcal infection should also receive an agent with activity against chlamydia.1 The recommended treatment regimen for pregnant women with uncomplicated gonorrhea is a single dose of ceftriaxone 250 mg intramuscularly (IM) or cefixime 400 mg orally, given in combination with azithromycin 1 g orally as a single dose (the recommended treatment for chlamydia during pregnancy). All patients should be tested for eradication of chlamydia 3 weeks after treatment completion.1,5
Genital Herpes: Genital herpes, a viral infection caused by herpes simplex virus types 1 and 2, afflicts more than 800,000 pregnant women annually in the United States.6,7 In more than 90% of cases of neonatal herpes (which affects the skin, eye, mouth, or central nervous system or causes disseminated infection), the infant acquires the infection during vaginal birth, when it is exposed to infected secretions in the mother’s genital tract. Therefore, to prevent neonatal infection, cesarean delivery (CD) is recommended for women who have active genital lesions or prodromal symptoms suggestive of genital herpes at the time of labor.7 Additionally, during the third trimester, women without genital herpes should abstain from intercourse with partners known or suspected to have genital herpes and to abstain from receptive oral sex with partners known or suspected to have orolabial herpes.1
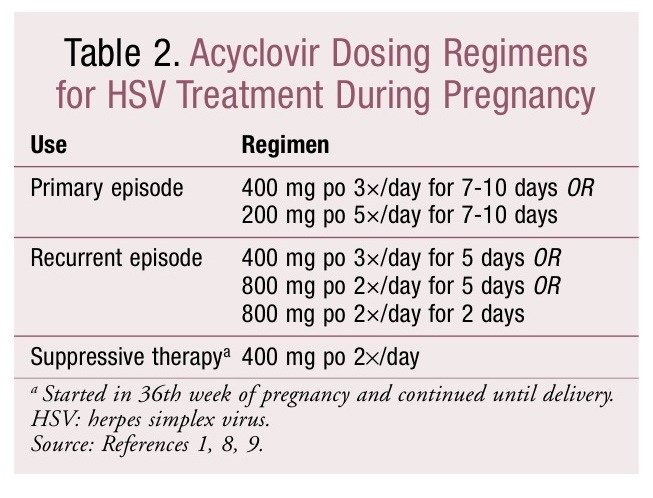
The safety of acyclovir, valacyclovir, and famciclovir for herpes treatment during pregnancy has not been established, and none of these agents is approved for use during pregnancy.1,8 There is no increased risk of major birth defects with the use of acyclovir during pregnancy, and taking acyclovir between the 36th week of pregnancy and delivery reduces the frequency of disease recurrence and the need for CD.1,8,9 Therefore, the CDC treatment guidelines for STDs recommend the use of acyclovir over other antivirals during pregnancy.1 TABLE 2 lists common oral dosing regimens for acyclovir. IV acyclovir may be used for severe infections.1
UTIs
UTIs—whether asymptomatic or symptomatic—are the most common bacterial infection in pregnancy, occurring in approximately 20% of patients.10,11 Asymptomatic bacteriuria (ASB) is the presence of more than 100,000 colony-forming units of bacteria per mL of urine from two consecutive urine cultures, occurring in the absence of UTI symptoms.12 Cystitis typically presents with urinary urgency and/or frequency, dysuria, or hematuria, whereas pyelonephritis usually involves systemic symptoms such as fever, costovertebral angle tenderness, flank pain, and nausea and vomiting, in addition to cystitis symptoms.10,13
Of the 2% to 10% of pregnant women with ASB, 15% to 45% will develop pyelonephritis if it is not treated.11 This is because many physiologic changes occur during pregnancy that ultimately result in stasis of urine in the ureters or bladder, increasing the risk of infection.11 It is important to prevent pyelonephritis in pregnancy, since it may cause maternal or perinatal complications (i.e., premature delivery, low birthweight, or fetal death).11,14,15 Therefore, patients should be screened for bacteriuria via urine culture at least once in early pregnancy (typically at 12-16 weeks’ gestation or first prenatal visit), with antibiotic treatment in the event of positive results.12,14 Some experts advocate repeated urine screening in each trimester in order to improve detection of ASB.10,13
The most common microbial etiology of UTI in pregnancy is Escherichia coli.10 Additional pathogens include other gram-negative rods of the Enterobacteriaceae family and group B streptococci (GBS).10,13 Quantitative urine culture is the gold standard for diagnosis of UTI during pregnancy, but if it is unavailable, local resistance patterns should be used to select an antibiotic with activity against E coli.10,11 Commonly used antimicrobial regimens for ASB or cystitis include a 3- to 7-day course of nitrofurantoin and use of a first-generation cephalosporin, such as cephalexin.10,11,12 A single dose of fosfomycin may also be considered, although there is limited experience with the use of this agent during pregnancy.12
Pyelonephritis has historically been managed by initial hospitalization for IV fluids and IV antibiotics (typically with a penicillin or cephalosporin active against E coli), followed by a 2-week course of oral antibiotics. An aminoglycoside may be used alone or in combination with ampicillin for severe cases; however, this agent is classified as Pregnancy Category C and may cause fetal ototoxicity. Recent evidence indicates that pregnant women with pyelonephritis who are at less than 24 weeks’ gestation, are otherwise healthy, are not having recurrent infections, and do not have fever, nausea, vomiting, or signs of sepsis may be considered for outpatient therapy. A patient meeting these criteria may be managed during an observation period in the emergency department, at which time laboratory values are drawn and IV hydration and an IM dose of ceftriaxone are administered. The patient must have a follow-up visit 24 hours later, at which time an additional dose of ceftriaxone is given and clinical condition is assessed. If the patient’s clinical condition has improved, she may receive oral therapy. A commonly used regimen is cephalexin 500 mg four times daily for 10 days. For patients who remain symptomatic, IM ceftriaxone may be continued daily for a maximum of 5 days.11,15
After completion of any course of antibiotics for the treatment of pyelonephritis, a urine culture should be performed to confirm resolution of bacteriuria. Monthly urine cultures are recommended for surveillance purposes.11 Because recurrent pyelonephritis occurs in 6% to 8% of pregnant women, antibiotic prophylaxis with nitrofurantoin 100 mg orally daily or cephalexin 250 to 500 mg orally daily is recommended for all patients for the duration of pregnancy through 4 to 6 weeks postpartum.11,15
GBSD
GBSD is the primary infectious cause of neonatal morbidity and mortality in the United States. The main microbial etiology of GBSD is Streptococcus agalactiae, a gram-positive organism that asymptomatically colonizes the vagina or rectum. S agalactiae occurs in approximately 10% to 30% of pregnant women. Annually, GBS causes about 1,200 cases of invasive disease (particularly sepsis and pneumonia), typically within the first 24 to 48 hours of the infant’s life.16
The primary risk factor for infant GBSD is intrapartum GBS colonization in the mother. The newborn becomes infected through exposure to GBS, which may ascend into the amniotic fluid. The child may also be exposed to GBS during passage through the birth canal.16
Two main strategies are recommended to prevent GBSD in newborns: routine testing of women late in pregnancy and the use of antibiotics during labor.17 All patients between 35 and 37 weeks’ gestation should be screened for GBS colonization with a vaginal or rectal swab.16 Intrapartum antibiotic prophylaxis is recommended for patients with a positive screening test and for those who previously delivered an infant with GBSD or have GBS bacteriuria in the current pregnancy. It also is advised in patients with unknown GBS colonization status who deliver an infant at less than 37 weeks’ gestation, have a temperature of at least 100.4°F during labor, or have rupture of membranes for 18 hours or more. IV PCG at a dosage of 5 million U once, followed by 2.5 to 3 million U every 4 hours until delivery, is recommended first-line therapy. IV ampicillin at a dosage of 2 g once, followed by 1 g every 4 hours until delivery, is considered an acceptable alternative to PCG. Patients with a history of nonsevere allergy to penicillins may be managed with cefazolin, whereas those with a severe reaction to penicillins may be treated with clindamycin or vancomycin, depending upon the infecting microorganism’s susceptibility.16
Listeriosis
Listeriosis is caused by Listeria monocytogenes, a gram-positive bacterium that is normally found in soil and water.18 This infection occurs primarily in elderly or immunocompromised patients, newborns, and pregnant women.18,19 Pregnant women with listeriosis may be asymptomatic or experience merely fatigue or muscle aches. The infection can be passed to the fetus through the placenta or during passage through the birth canal, potentially causing miscarriage, stillbirth, premature delivery, or neonatal meningitis.18,20
The primary route by which listeriosis occurs is the ingestion of food contaminated with the bacterium. All pregnant women should be counseled to eat only fully cooked meats, thoroughly washed vegetables, or pasteurized milk, and to avoid certain cheeses (see TABLE 3 for more details).19,21
Toxoplasmosis
Toxoplasmosis is caused by the parasite Toxoplasma gondii. Although pregnant women with this infection may be asymptomatic, the infection can be passed to the fetus through the placenta, potentially causing congenital disease leading to infant death or to malformation, mental retardation, deafness, or blindness (either at birth or later in life).22,23
A pregnant woman may become infected with toxoplasmosis through foodborne exposure, environmental exposure, or animal transmission.22,24 With foodborne transmission, the primary route of infection is ingestion of meat containing tissue cysts of the parasite. Since cysts are immediately killed at 67°C, pregnant women should be counseled to avoid eating undercooked meat or meat that could be contaminated by utensils or cutting boards that have been in contact with raw or contaminated meat.24 Oocyst forms of the parasite may be present in the environment. A person may also become infected after accidental ingestion of soil that is contaminated with oocyst forms of the parasite. Therefore, pregnant women should wear gloves while gardening, wash their hands after gardening, and wash fruits, herbs, and vegetables prior to consumption.22,24
Cats play a major role in the transmission of infection to humans. After a cat eats a rodent or other small animal infected with toxoplasmosis, the oocyst form of the parasite is shed in its feces for up to 3 weeks after infection.22 Therefore, a pregnant woman may become infected with toxoplasmosis by unintentionally touching her mouth after changing the litter box.23 Patients should be advised to keep their own cats indoors; feed their cat commercial dry or canned food rather than raw or undercooked meats; avoid stray cats; and avoid obtaining a new cat while pregnant. If possible, the patient should avoid changing cat litter; if this is not possible, she should wear disposable gloves during the task and wash her hands with soap and water immediately afterward.23,24 Since the parasite does not become infectious until 1 to 5 days after it is shed in a cat’s feces, changing the litter box daily will prevent the spread of infection.23
Conclusion
Infections are common during pregnancy. Pharmacists are in a key position to improve both maternal and fetal patient outcomes by counseling the mother on appropriate preventive measures, recommending optimal antimicrobial therapy regimens, and monitoring for adverse effects. Pharmacists must actively work with patients and health care providers to ensure the proper prevention and treatment of infections during pregnancy.
REFERENCES
1. Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
2. De Santis M, De Luca C, Mappa I, et al. Syphilis infection during pregnancy: fetal risks and clinical management. Infect Dis Obstet Gynecol. 2012;2012:430585.
3. Oswal S, Lyons G. Syphilis in pregnancy. Contin Educ Anaesth Crit Care Pain. 2008;8:224-227.
4. Blatt AJ, Lieberman JM, Hoover DR, Kaufman HW. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-e8.
5. Ruhl C. Update on chlamydia and gonorrhea screening during pregnancy. Nurs Womens Health. 2013;17:143-146.
6. CDC. STDs & pregnancy—CDC fact sheet. www.cdc.gov/std/pregnancy/STDs-and-pregnancy-fact-sheet-February2012.pdf. Accessed May 1, 2013.
7. Gardella C, Brown ZA. Managing genital herpes infections in pregnancy. Cleve Clin J Med. 2007;74:217-224.
8. Straface G, Selmin A, Zanardo V, et al. Herpes simplex virus infection in pregnancy. Infect Dis Obstet Gynecol. 2012;2012:385697.
9. Sheffield JS, Hollier LM, Hill JB, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
10. Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest. 2008;38(suppl 2):50-57.
11. Torres M, Moayedi S. Gynecologic and other infections in pregnancy. Emerg Med Clin North Am. 2012;30:869-884.
12. Lumbiganon P, Laopaiboon M, Thinkhamrop J. Screening and treating asymptomatic bacteriuria in pregnancy. Curr Opin Obstet Gynecol. 2010;22:95-99.
13. Le J, Briggs GG, McKeown A, Bustillo G. Urinary tract infections during pregnancy. Ann Pharmacother. 2004;38:1692-1701.
14. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
15. Jolley JA, Wing DA. Pyelonephritis in pregnancy: an update on treatment options for optimal outcomes. Drugs. 2010;70:1643-1655.
16. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1-36.
17. CDC. Group B strep (GBS). Prevention in newborns. www.cdc.gov/groupbstrep/about/prevention.html. Accessed May 1, 2013.
18. CDC. Listeria (listeriosis). Definition. www.cdc.gov/listeria/definition.html. Accessed May 1, 2013.
19. CDC. Listeriosis (listeria) and pregnancy. www.cdc.gov/pregnancy/infections-Listeria.html. Accessed May 1, 2013.
20. Bubonja-Sonje M, Mustac E, Brunn A, et al. Listeriosis in pregnancy: case report and retrospective study. J Matern Fetal Neonatal Med. 2013;26:321-323.
21. USDA Food Safety and Inspection Service. Protect your baby and yourself from listeriosis. www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/foodborne-illness-and-disease/protect-your-baby-and-yourself-from-listeriosis/CT_Index. Accessed May 1, 2013.
22. Di Mario S, Basevi V, Gagliotti C, et al. Prenatal education for congenital toxoplasmosis. Cochrane Database Syst Rev. 2013;(2):CD006171.
23. CDC. Parasites—toxoplasmosis (Toxoplasma infection). Pregnant women. www.cdc.gov/parasites/toxoplasmosis/gen_info/pregnant.html. Accessed May 1, 2013.
24. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264-296.
- See more at: http://www.uspharmacist.com/content/d/feature/c/43013/#sthash.sE3nVlzT.dpuf







 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 